Effect of Alkali-Free Synthesis and Post-Synthetic Treatment on Acid Sites in Beta Zeolites
- PMID: 32731634
- PMCID: PMC7435978
- DOI: 10.3390/molecules25153434
Effect of Alkali-Free Synthesis and Post-Synthetic Treatment on Acid Sites in Beta Zeolites
Abstract
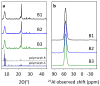
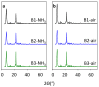
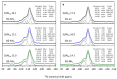
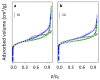
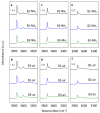
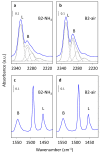
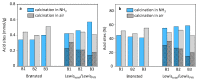
Beta zeolites with Si/Al around 14 were prepared using three new alkali-free synthesis methods based on the application of amorphous aluminosilicate precursor and calcined in ammonia or air. All samples exhibit structural and textural properties of standard beta zeolite. Comprehensive study by 27Al and 29Si MAS NMR, together with FTIR adsorption of d3-acetonitrile and pyridine were used to characterize the influence of both the synthesis and calcination procedure on the framework Al atoms and related Brønsted and Lewis acid sites. While calcination in ammonia preserves all framework Al atoms, calcination in air results in 15% release of framework Al, but without restrictions of the accessibility of the beta zeolite channel system for bulky pyridine molecules. Terminal (SiO)3AlOH groups present in the hydrated zeolites were suggested as a precursor of framework Al-Lewis sites. Surprisingly, the mild dealumination of the air-calcined zeolites result in an increase of the concentration of Brønsted acid sites and a decrease of the total concentration of Lewis sites with the formation of the extra-framework ones.
Keywords: Brønsted acid sites; acid sites; air calcination; alkali-free synthesis; ammonia calcination; beta zeolite; de-templating; extra-framework Al-Lewis sites; framework Al-Lewis sites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Roy S., Hegde M.S., Madras G. Catalysis for NOx abatement. Appl. Energy. 2009;86:2283–2297. doi: 10.1016/j.apenergy.2009.03.022. - DOI
-
- Shan W., Song H. Catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. Catal. Sci. Technol. 2015;5:4280–4288. doi: 10.1039/C5CY00737B. - DOI
-
- Casci J.L., Cundy C.S., Barrer R.M. Hydrothermal Chemistry of Zeolites. Academic Press; London, UK: New York, NY, USA: 1982. p. 360. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

