Microvesicles in Cancer: Small Size, Large Potential
- PMID: 32731639
- PMCID: PMC7432491
- DOI: 10.3390/ijms21155373
Microvesicles in Cancer: Small Size, Large Potential
Abstract
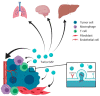
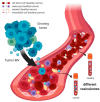
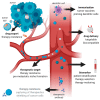
Extracellular vesicles (EV) are secreted by all cell types in a tumor and its microenvironment (TME), playing an essential role in intercellular communication and the establishment of a TME favorable for tumor invasion and metastasis. They encompass a variety of vesicle populations, among them the well-known endosomal-derived small exosomes (Exo), but also larger vesicles (diameter > 100 nm) that are shed directly from the plasma membrane, the so-called microvesicles (MV). Increasing evidence suggests that MV, although biologically different, share the tumor-promoting features of Exo in the TME. Due to their larger size, they can be readily harvested from patients' blood and characterized by routine methods such as conventional flow cytometry, exploiting the plethora of molecules expressed on their surface. In this review, we summarize the current knowledge about the biology and the composition of MV, as well as their role within the TME. We highlight not only the challenges and potential of MV as novel biomarkers for cancer, but also discuss their possible use for therapeutic intervention.
Keywords: biomarker; cancer; microvesicles; therapy; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Characterization of single microvesicles in plasma from glioblastoma patients.Neuro Oncol. 2019 May 6;21(5):606-615. doi: 10.1093/neuonc/noy187. Neuro Oncol. 2019. PMID: 30561734 Free PMC article.
-
Tumor-cell-derived microvesicles as carriers of molecular information in cancer.Curr Opin Oncol. 2013 Jan;25(1):66-75. doi: 10.1097/CCO.0b013e32835b7c81. Curr Opin Oncol. 2013. PMID: 23165142 Review.
-
Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations.Biomed Microdevices. 2014 Dec;16(6):869-77. doi: 10.1007/s10544-014-9891-z. Biomed Microdevices. 2014. PMID: 25342569 Free PMC article.
-
Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer.Int J Mol Sci. 2016 Feb 6;17(2):175. doi: 10.3390/ijms17020175. Int J Mol Sci. 2016. PMID: 26861306 Free PMC article. Review.
-
Microvesicles and chemokines in tumor microenvironment: mediators of intercellular communications in tumor progression.Mol Cancer. 2019 Mar 30;18(1):50. doi: 10.1186/s12943-019-0973-7. Mol Cancer. 2019. PMID: 30925930 Free PMC article. Review.
Cited by
-
Canine B Cell Lymphoma- and Leukemia-Derived Extracellular Vesicles Moderate Differentiation and Cytokine Production of T and B Cells In Vitro.Int J Mol Sci. 2022 Aug 29;23(17):9831. doi: 10.3390/ijms23179831. Int J Mol Sci. 2022. PMID: 36077229 Free PMC article.
-
Extracellular Vesicles-A New Potential Player in the Immunology of Renal Cell Carcinoma.J Pers Med. 2022 May 10;12(5):772. doi: 10.3390/jpm12050772. J Pers Med. 2022. PMID: 35629194 Free PMC article. Review.
-
Extracellular Vesicles and Epidermal Growth Factor Receptor Activation: Interplay of Drivers in Cancer Progression.Cancers (Basel). 2023 May 30;15(11):2970. doi: 10.3390/cancers15112970. Cancers (Basel). 2023. PMID: 37296932 Free PMC article. Review.
-
Liquid Biopsy as a Diagnostic and Monitoring Tool in Glioblastoma.Medicina (Kaunas). 2025 Apr 13;61(4):716. doi: 10.3390/medicina61040716. Medicina (Kaunas). 2025. PMID: 40283007 Free PMC article. Review.
-
A machine-learning approach for pancreatic neoplasia classification based on plasma extracellular vesicles.Front Oncol. 2025 Apr 25;15:1540195. doi: 10.3389/fonc.2025.1540195. eCollection 2025. Front Oncol. 2025. PMID: 40352592 Free PMC article.
References
-
- Yáñez-Mó M., Siljander P.R.-M., Andreu Z., Bedina Zavec A., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

