Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome
- PMID: 32732245
- PMCID: PMC7509521
- DOI: 10.1136/annrheumdis-2020-217712
Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome
Abstract
Objectives: The outbreak of COVID-19 posed the issue of urgently identifying treatment strategies. Colchicine was considered for this purpose based on well-recognised anti-inflammatory effects and potential antiviral properties. In the present study, colchicine was proposed to patients with COVID-19, and its effects compared with 'standard-of-care' (SoC).
Methods: In the public hospital of Esine, northern Italy, 140 consecutive inpatients, with virologically and radiographically confirmed COVID-19 admitted in the period 5-19 March 2020, were treated with 'SoC' (hydroxychloroquine and/or intravenous dexamethasone; and/or lopinavir/ritonavir). They were compared with 122 consecutive inpatients, admitted between 19 March and 5 April 2020, treated with colchicine (1 mg/day) and SoC (antiviral drugs were stopped before colchicine, due to potential interaction).
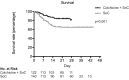
Results: Patients treated with colchicine had a better survival rate as compared with SoC at 21 days of follow-up (84.2% (SE=3.3%) vs 63.6% (SE=4.1%), p=0.001). Cox proportional hazards regression survival analysis showed that a lower risk of death was independently associated with colchicine treatment (HR=0.151 (95% CI 0.062 to 0.368), p<0.0001), whereas older age, worse PaO2/FiO2, and higher serum levels of ferritin at entry were associated with a higher risk.
Conclusion: This proof-of-concept study may support the rationale of use of colchicine for the treatment of COVID-19. Efficacy and safety must be determined in controlled clinical trials.
Keywords: anti-inflammatory agents, non-steroidal; antirheumatic agents; communicable diseases, imported; inflammation; therapeutics.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
Anti-inflammatory action of colchicine in hospitalised patients with COVID-19. Response to: 'Colchicine treatment in community healthcare setting to prevent severe COVID-19' by Della-Torre et al.Ann Rheum Dis. 2022 Oct;81(10):e199. doi: 10.1136/annrheumdis-2020-218806. Epub 2020 Aug 27. Ann Rheum Dis. 2022. PMID: 32855151 No abstract available.
-
Colchicine treatment in community healthcare setting to prevent severe COVID-19.Ann Rheum Dis. 2022 Oct;81(10):e198. doi: 10.1136/annrheumdis-2020-218759. Epub 2020 Aug 27. Ann Rheum Dis. 2022. PMID: 32855152 No abstract available.
-
Response to: 'Correspondence on 'Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome'' by Kawada.Ann Rheum Dis. 2023 Apr;82(4):e78. doi: 10.1136/annrheumdis-2020-219787. Epub 2021 Jan 27. Ann Rheum Dis. 2023. PMID: 33504480 No abstract available.
-
Correspondence on 'Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome'.Ann Rheum Dis. 2023 Apr;82(4):e77. doi: 10.1136/annrheumdis-2020-219771. Epub 2021 Jan 27. Ann Rheum Dis. 2023. PMID: 33504481 No abstract available.
References
-
- Ferro F, Elefante E, Baldini C, et al. . COVID-19: the new challenge for rheumatologists. Clin Exp Rheumatol 2020;38:175–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous