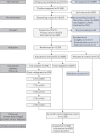
Earlier diagnosis of lung cancer in a randomised trial of an autoantibody blood test followed by imaging
- PMID: 32732334
- PMCID: PMC7806972
- DOI: 10.1183/13993003.00670-2020
Earlier diagnosis of lung cancer in a randomised trial of an autoantibody blood test followed by imaging
Abstract
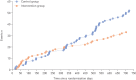
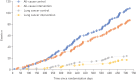
The EarlyCDT-Lung test is a high-specificity blood-based autoantibody biomarker that could contribute to predicting lung cancer risk. We report on the results of a phase IV biomarker evaluation of whether using the EarlyCDT-Lung test and any subsequent computed tomography (CT) scanning to identify those at high risk of lung cancer reduces the incidence of patients with stage III/IV/unspecified lung cancer at diagnosis compared with the standard clinical practice at the time the study began.The Early Diagnosis of Lung Cancer Scotland (ECLS) trial was a randomised controlled trial of 12 208 participants at risk of developing lung cancer in Scotland in the UK. The intervention arm received the EarlyCDT-Lung test and, if test-positive, low-dose CT scanning 6-monthly for up to 2 years. EarlyCDT-Lung test-negative and control arm participants received standard clinical care. Outcomes were assessed at 2 years post-randomisation using validated data on cancer occurrence, cancer staging, mortality and comorbidities.At 2 years, 127 lung cancers were detected in the study population (1.0%). In the intervention arm, 33 out of 56 (58.9%) lung cancers were diagnosed at stage III/IV compared with 52 out of 71 (73.2%) in the control arm. The hazard ratio for stage III/IV presentation was 0.64 (95% CI 0.41-0.99). There were nonsignificant differences in lung cancer and all-cause mortality after 2 years.ECLS compared EarlyCDT-Lung plus CT screening to standard clinical care (symptomatic presentation) and was not designed to assess the incremental contribution of the EarlyCDT-Lung test. The observation of a stage shift towards earlier-stage lung cancer diagnosis merits further investigations to evaluate whether the EarlyCDT-Lung test adds anything to the emerging standard of low-dose CT.
Trial registration: ClinicalTrials.gov NCT01925625.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: F.M. Sullivan reports grants from Oncimmune and the Scottish Government Health and Social Care Directorate of the Chief Scientist Office, during the conduct of the study. Conflict of interest: F.S. Mair reports grants from Oncimmune and the Scottish Government Health and Social Care Directorate of the Chief Scientist Office, during the conduct of the study. Conflict of interest: W. Anderson has nothing to disclose. Conflict of interest: P. Armory reports grants from Oncimmune and the Scottish Government Health and Social Care Directorate of the Chief Scientist Office, during the conduct of the study. Conflict of interest: A. Briggs reports grants from the Scottish Government Health and Social Care Directorate of the Chief Scientist Office and Oncimmune, during the conduct of the study. Conflict of interest: C. Chew has nothing to disclose. Conflict of interest: A. Dorward has nothing to disclose. Conflict of interest: J. Haughney has nothing to disclose. Conflict of interest: F. Hogarth reports grants from the Scottish Government Health and Social Care Directorate of the Chief Scientist Office and from Oncimmune, during the conduct of the study. Conflict of interest: D. Kendrick has nothing to disclose. Conflict of interest: R. Littleford reports grants from the Scottish Government Health and Social Care Directorate of the Chief Scientist Office and Oncimmune, during the conduct of the study. Conflict of interest: A. McConnachie reports grants from Oncimmune and the Scottish Government Health and Social Care Directorate of the Chief Scientist Office, during the conduct of the study. Conflict of interest: C. McCowan has nothing to disclose. Conflict of interest: N. McMeekin reports grants from Oncimmune and the Scottish Government Health and Social Care Directorate of the Chief Scientist Office, during the conduct of the study. Conflict of interest: M. Patel has nothing to disclose. Conflict of interest: P. Rauchhaus reports grants from Oncimmune and the Scottish Government Health and Social Care Directorate of the Chief Scientist Office, during the conduct of the study. Conflict of interest: L. Ritchie has nothing to disclose. Conflict of interest: C. Robertson reports personal fees and other funding from Oncimmune, outside the study. Conflict of interest: J. Robertson reports other funding from Oncimmune, during the conduct of the study; and other funding from Oncimmune, outside the study. J. Robertson was a founder of Oncimmune, a company spun out from the University of Nottingham based on his academic research. Between 2003 and 2013 he was Chief Scientific Officer of Oncimmune and a Director of the company. During this time, he was responsible for the original drafting of the ECLS protocol. Since 2013 he has had no involvement in the science or management of the company. He has been and remains a shareholder in the company. Conflict of interest: J. Robles-Zurita reports grants from the Scottish Government Health and Social Care Directorate of the Chief Scientist Office and Oncimmune, during the conduct of the study. Conflict of interest: J. Sarvesvaran has nothing to disclose. Conflict of interest: H. Sewell reports other funding from Oncimmune, outside the submitted work; and was an external member of the Oncimmune Scientific Advisory Board from 2006 to 2013. Conflict of interest: M. Sproule has nothing to disclose. Conflict of interest: T. Taylor reports grants, nonfinancial support and other funding from Oncimmune, grants and personal fees from the Chief Scientist Office for Scotland, and grants and nonfinancial support from the Scottish Government, outside the submitted work. Conflict of interest: A. Tello reports grants from Oncimmune, during the conduct of the study. Conflict of interest: S. Treweek reports grants from Oncimmune and the Scottish Government Health and Social Care Directorate of the Chief Scientist Office, during the conduct of the study. Conflict of interest: K. Vedhara has nothing to disclose. Conflict of interest: S. Schembri reports grants from Oncimmune and the Scottish Government Health and Social Care Directorate of the Chief Scientist Office, during the conduct of the study; and nonfinancial support from GlaxoSmithKline and AstraZeneca, outside the submitted work.
Figures
Comment in
-
Biomarkers in lung cancer screening: the importance of study design.Eur Respir J. 2021 Jan 14;57(1):2004367. doi: 10.1183/13993003.04367-2020. Print 2021 Jan. Eur Respir J. 2021. PMID: 33446580 Free PMC article. No abstract available.
References
-
- Allemani C, Matsuda T, Di Carlo V, et al. . Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–1075. doi:10.1016/S0140-6736(17)33326-3 - DOI - PMC - PubMed