Paraventricular hypothalamus mediates diurnal rhythm of metabolism
- PMID: 32732906
- PMCID: PMC7393104
- DOI: 10.1038/s41467-020-17578-7
Paraventricular hypothalamus mediates diurnal rhythm of metabolism
Abstract
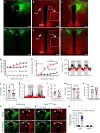
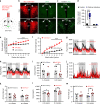
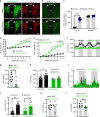
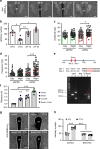
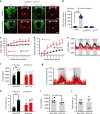
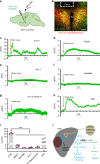
Defective rhythmic metabolism is associated with high-fat high-caloric diet (HFD) feeding, ageing and obesity; however, the neural basis underlying HFD effects on diurnal metabolism remains elusive. Here we show that deletion of BMAL1, a core clock gene, in paraventricular hypothalamic (PVH) neurons reduces diurnal rhythmicity in metabolism, causes obesity and diminishes PVH neuron activation in response to fast-refeeding. Animal models mimicking deficiency in PVH neuron responsiveness, achieved through clamping PVH neuron activity at high or low levels, both show obesity and reduced diurnal rhythmicity in metabolism. Interestingly, the PVH exhibits BMAL1-controlled rhythmic expression of GABA-A receptor γ2 subunit, and dampening rhythmicity of GABAergic input to the PVH reduces diurnal rhythmicity in metabolism and causes obesity. Finally, BMAL1 deletion blunts PVH neuron responses to external stressors, an effect mimicked by HFD feeding. Thus, BMAL1-driven PVH neuron responsiveness in dynamic activity changes involving rhythmic GABAergic neurotransmission mediates diurnal rhythmicity in metabolism and is implicated in diet-induced obesity.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Disrupted hypothalamic CRH neuron responsiveness contributes to diet-induced obesity.EMBO Rep. 2020 Jul 3;21(7):e49210. doi: 10.15252/embr.201949210. Epub 2020 May 27. EMBO Rep. 2020. PMID: 32462726 Free PMC article.
-
The Paraventricular Hypothalamus Regulates Satiety and Prevents Obesity via Two Genetically Distinct Circuits.Neuron. 2019 May 8;102(3):653-667.e6. doi: 10.1016/j.neuron.2019.02.028. Epub 2019 Mar 14. Neuron. 2019. PMID: 30879785 Free PMC article.
-
SH2B1 Defends Against Energy Imbalance, Obesity, and Metabolic Disease via a Paraventricular Hypothalamus→Dorsal Raphe Nucleus Neurocircuit.Adv Sci (Weinh). 2024 Aug;11(31):e2400437. doi: 10.1002/advs.202400437. Epub 2024 Jun 17. Adv Sci (Weinh). 2024. PMID: 38885417 Free PMC article.
-
Effects of BMAL1 Manipulation on the Brain's Master Circadian Clock and Behavior.Yale J Biol Med. 2019 Jun 27;92(2):251-258. eCollection 2019 Jun. Yale J Biol Med. 2019. PMID: 31249486 Free PMC article. Review.
-
[The roles of clock genes in obesity].Nihon Rinsho. 2013 Feb;71(2):244-8. Nihon Rinsho. 2013. PMID: 23631200 Review. Japanese.
Cited by
-
Reciprocal Interactions between Circadian Clocks, Food Intake, and Energy Metabolism.Biology (Basel). 2023 Mar 31;12(4):539. doi: 10.3390/biology12040539. Biology (Basel). 2023. PMID: 37106739 Free PMC article. Review.
-
Discrete TrkB-expressing neurons of the dorsomedial hypothalamus regulate feeding and thermogenesis.Proc Natl Acad Sci U S A. 2021 Jan 26;118(4):e2017218118. doi: 10.1073/pnas.2017218118. Proc Natl Acad Sci U S A. 2021. PMID: 33468645 Free PMC article.
-
Dietary Conjugated Linoleic Acid Reduces Body Weight and Fat in Snord116m+/p- and Snord116m-/p- Mouse Models of Prader-Willi Syndrome.Nutrients. 2022 Feb 18;14(4):860. doi: 10.3390/nu14040860. Nutrients. 2022. PMID: 35215509 Free PMC article.
-
Satiety Associated with Calorie Restriction and Time-Restricted Feeding: Central Neuroendocrine Integration.Adv Nutr. 2022 Jun 1;13(3):758-791. doi: 10.1093/advances/nmac011. Adv Nutr. 2022. PMID: 35134815 Free PMC article. Review.
-
Light-induced epigenetic modifications in the hypothalamus during avian embryonic development enhance phenotypic plasticity.Front Cell Dev Biol. 2025 Jun 26;13:1573705. doi: 10.3389/fcell.2025.1573705. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40641602 Free PMC article.
References
-
- Bechtold DA, Loudon AS. Hypothalamic clocks and rhythms in feeding behaviour. Trends Neurosci. 2013;36:74–82. - PubMed
-
- Satinoff E, Prosser RA. Suprachiasmatic nuclear lesions eliminate circadian rhythms of drinking and activity, but not of body temperature, in male rats. J. Biol. Rhythms. 1988;3:1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

