Fluorescence-assisted sequential insertion of transgenes (FASIT): an approach for increasing specific productivity in mammalian cells
- PMID: 32732973
- PMCID: PMC7392891
- DOI: 10.1038/s41598-020-69709-1
Fluorescence-assisted sequential insertion of transgenes (FASIT): an approach for increasing specific productivity in mammalian cells
Abstract
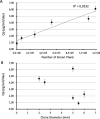
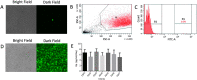
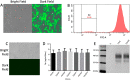
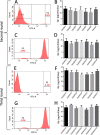
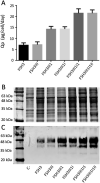
Currently, the generation of cell lines for the production of recombinant proteins has the limitation of unstable gene expression due to the repeat-induced gene silencing or the loss of transgene copies resulting from recombination events. In this work, we developed a new strategy based on the sequential insertion of transgenes for generating stable clones producing high levels of a chimeric human follicle-stimulating hormone (hscFSH). Gene insertion was done by transducing HEK-293 cells with a lentiviral vector containing a bicistronic transcriptional unit for expressing hscFSH and GFP genes. Clone selection was performed by flow cytometry coupled to cell sorting, and the GFP gene was further removed by CRE-mediated site-specific recombination. High-producing clones of hscFSH were obtained after three rounds of lentiviral transduction. Expression levels increased in a step-wise manner from 7 to 23 pg/cell/day, with a relatively constant rate of 7 pg/cell/day in each round of transduction. The GFP gene was successfully removed from the cell genome without disturbing the hscFSH gene expression. Clones generated using this approach showed stable expression levels for more than two years. This is the first report describing the sequential insertion of transgenes as an alternative for increasing the expression levels of transformed cell lines. The methodology described here could notably impact on biotechnological industry by improving the capacity of mammalian cells to produce biopharmaceuticals.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

