Simultaneous time-varying viscosity, elasticity, and mass measurements of single adherent cancer cells across cell cycle
- PMID: 32733047
- PMCID: PMC7393350
- DOI: 10.1038/s41598-020-69638-z
Simultaneous time-varying viscosity, elasticity, and mass measurements of single adherent cancer cells across cell cycle
Abstract
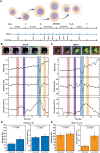
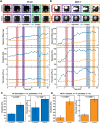
Biophysical studies on single cells have linked cell mechanics to physiology, functionality and disease. Evaluation of mass and viscoelasticity versus cell cycle can provide further insights into cell cycle progression and the uncontrolled proliferation of cancer. Using our pedestal microelectromechanical systems resonant sensors, we have developed a non-contact interferometric measurement technique that simultaneously tracks the dynamic changes in the viscoelastic moduli and mass of adherent colon (HT-29) and breast cancer (MCF-7) cells from the interphase through mitosis and then to the cytokinesis stages of their growth cycle. We show that by combining three optomechanical parameters in an optical path length equation and a two-degree-of-freedom model, we can simultaneously extract the viscoelasticity and mass as a function of the nano-scaled membrane fluctuation of each adherent cell. Our measurements are able to discern between soft and stiff cells across the cell cycle and demonstrated sharp viscoelastic changes due to cortical stiffening around mitosis. Cell rounding before division can be detected by measurement of mechanical coupling between the cells and the sensors. Our measurement device and method can provide for new insights into the mechanics of single adherent cells versus time.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Corbin EA, Kong F, Lim T, King PW, Bashir R. Biophysical properties of human breast cancer cells measured using silicon MEMS resonators and atomic force microscopy. Lab Chip. 2014;15(3):839–47. - PubMed
-
- Cross SE, Jin Y-C, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007;2:780–783. - PubMed
-
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer. 2004;4:118. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

