Ketogenic Diet Provided During Three Months Increases KCC2 Expression but Not NKCC1 in the Rat Dentate Gyrus
- PMID: 32733191
- PMCID: PMC7358437
- DOI: 10.3389/fnins.2020.00673
Ketogenic Diet Provided During Three Months Increases KCC2 Expression but Not NKCC1 in the Rat Dentate Gyrus
Abstract
Ketogenic diet, a high fat and low carbohydrate diet, has been used as a non-pharmacological treatment in refractory epilepsy since 1920. In recent years, it has demonstrated to be effective in the treatment of numerous neurological and non-neurological diseases. Some neurological and neuropsychiatric disorders are known to be caused by gamma-aminobutyric acid (GABA)-mediated neurotransmission dysfunction. The strength and polarity of GABA-mediated neurotransmission are determined by the intracellular chloride concentration, which in turn is regulated by cation-chloride cotransporters NKCC1 and KCC2. Currently, it is unknown if the effect of ketogenic diet is due to the modulation of these cotransporters. Thus, we analyzed the effect of a ketogenic diet on the cation-chloride cotransporters expression in the dentate gyrus. We estimated the total number of NKCC1 immunoreactive (NKCC1-IR) neuronal and glial cells by stereology and determined KCC2 labeling intensity by densitometry in the molecular and granule layers as well as in the hilus of dentate gyrus of rats fed with normal or ketogenic diet for 3 months. The results indicated that ketogenic diet provided during 3 months increased KCC2 expression, but not NKCC1 in the dentate gyrus of the rat. The significant increase of KCC2 expression could explain, at least in part, the beneficial effect of ketogenic diet in the diseases where the GABAergic system is altered by increasing its inhibitory efficiency.
Keywords: KCC2; NKCC1; dentate gyrus; ketogenic diet; optical density; optical fractionator; rat.
Copyright © 2020 Granados-Rojas, Jerónimo-Cruz, Juárez-Zepeda, Tapia-Rodríguez, Tovar, Rodríguez-Jurado, Carmona-Aparicio, Cárdenas-Rodríguez, Coballase-Urrutia, Ruíz-García and Durán.
Figures
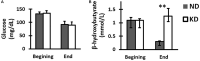



References
LinkOut - more resources
Full Text Sources

