Systems Biology Approaches to Understand the Host-Microbiome Interactions in Neurodegenerative Diseases
- PMID: 32733199
- PMCID: PMC7360858
- DOI: 10.3389/fnins.2020.00716
Systems Biology Approaches to Understand the Host-Microbiome Interactions in Neurodegenerative Diseases
Abstract
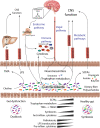
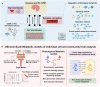
Neurodegenerative diseases (NDDs) comprise a broad range of progressive neurological disorders with multifactorial etiology contributing to disease pathophysiology. Evidence of the microbiome involvement in the gut-brain axis urges the interest in understanding metabolic interactions between the microbiota and host physiology in NDDs. Systems Biology offers a holistic integrative approach to study the interplay between the different biologic systems as part of a whole, and may elucidate the host-microbiome interactions in NDDs. We reviewed direct and indirect pathways through which the microbiota can modulate the bidirectional communication of the gut-brain axis, and explored the evidence of microbial dysbiosis in Alzheimer's and Parkinson's diseases. As the gut microbiota being strongly affected by diet, the potential approaches to targeting the human microbiota through diet for the stimulation of neuroprotective microbial-metabolites secretion were described. We explored the potential of Genome-scale metabolic models (GEMs) to infer microbe-microbe and host-microbe interactions and to identify the microbiome contribution to disease development or prevention. Finally, a systemic approach based on GEMs and 'omics integration, that would allow the design of sustainable personalized anti-inflammatory diets in NDDs prevention, through the modulation of gut microbiota was described.
Keywords: Alzheimer’s disease; Parkinson’s disease; biologic network; biomarker discovery; dietary therapy; microbiota-gut-brain axis; neurodegenerative diseases; systems biology.
Copyright © 2020 Rosario, Boren, Uhlen, Proctor, Aarsland, Mardinoglu and Shoaie.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

