Characterization of PF-6142, a Novel, Non-Catecholamine Dopamine Receptor D1 Agonist, in Murine and Nonhuman Primate Models of Dopaminergic Activation
- PMID: 32733245
- PMCID: PMC7358525
- DOI: 10.3389/fphar.2020.01005
Characterization of PF-6142, a Novel, Non-Catecholamine Dopamine Receptor D1 Agonist, in Murine and Nonhuman Primate Models of Dopaminergic Activation
Abstract
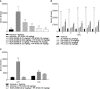
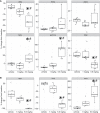
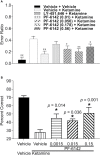
Selective activation of dopamine D1 receptors remains a promising pro-cognitive therapeutic strategy awaiting robust clinical investigation. PF-6142 is a key example from a recently disclosed novel series of non-catechol agonists and partial agonists of the dopamine D1/5 receptors (D1R) that exhibit pharmacokinetic (PK) properties suitable for oral delivery. Given their reported potential for functionally biased signaling compared to known catechol-based selective agonists, and the promising rodent PK profile of PF-6142, we utilized relevant in vivo assays in male rodents and male and female non-human primates (NHP) to evaluate the pharmacology of this new series. Studies in rodents showed that PF-6142 increased locomotor activity and prefrontal cortex acetylcholine release, increased time spent in wakefulness, and desynchronized the EEG, like known D1R agonists. D1R selectivity of PF-6142 was supported by lack of effect in D1R knock-out mice and blocked response in the presence of the D1R antagonist SCH-23390. Further, PF-6142 improved performance in rodent models of NMDA receptor antagonist-induced cognitive dysfunction, such as MK-801-disrupted paired-pulse facilitation, and ketamine-disrupted working memory performance in the radial arm maze. Similarly, PF-6142 reversed ketamine-induced deficits in NHP performing the spatial delayed recognition task. Of importance, PF-6142 did not alter the efficacy of risperidone in assays predictive of antipsychotic-like effect in rodents including pre-pulse inhibition and conditioned avoidance responding. These data support the continued development of non-catechol based D1R agonists for the treatment of cognitive impairment associated with brain disorders including schizophrenia.
Keywords: Parkinson’s disease; prefrontal cortex; pro-cognitive therapeutics; schizophrenia; working memory.
Copyright © 2020 Kozak, Kiss, Dlugolenski, Johnson, Gorczyca, Kuszpit, Harvey, Stolyar, Sukoff Rizzo, Hoffmann, Volfson, Hajós, Davoren, Abbott, Williams, Castner and Gray.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

