Glycosaminoglycans as Multifunctional Anti-Elastase and Anti-Inflammatory Drugs in Cystic Fibrosis Lung Disease
- PMID: 32733248
- PMCID: PMC7360816
- DOI: 10.3389/fphar.2020.01011
Glycosaminoglycans as Multifunctional Anti-Elastase and Anti-Inflammatory Drugs in Cystic Fibrosis Lung Disease
Abstract
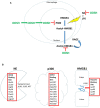
Neutrophil elastase (NE) is a major protease in the airways of patients with cystic fibrosis (CF) that activates airway inflammation by several mechanisms. NE stimulates epithelial toll like receptors (TLR) resulting in cytokine upregulation and release, upregulates MUC5AC, a major airway mucin, degrades both phagocytic receptors and opsonins resulting in both neutrophil and macrophage phagocytic failure, generates oxidative stress via extracellular generation and uptake of heme free iron, and activates other proteases. Altogether, these mechanisms create a significant inflammatory challenge that impairs innate immune function and results in airway remodeling. Currently, a major gap in our therapeutic approach to CF lung disease is the lack of an effective therapeutic strategy targeting active NE and its downstream pro-inflammatory sequelae. Polysulfated glycosaminoglycans (GAGs) are potent anti-elastase drugs that have additional anti-inflammatory properties. Heparin is a prototype of a glycosaminoglycan with both anti-elastase and anti-inflammatory properties. Heparin inhibits NE in an allosteric manner with high potency. Heparin also inhibits cathepsin G, blocks P-selectin and L-selectin, hinders ligand binding to the receptor for advanced glycation endproducts, and impedes histone acetyltransferase activity which dampens cytokine transcription and High Mobility Group Box 1 release. Furthermore, nebulized heparin treatment improves outcomes for patients with chronic obstructive pulmonary disease (COPD), asthma, acute lung injury and smoke inhalation. However, the anticoagulant activity of heparin is a potential contraindication for this therapy to be developed for CF lung disease. Therefore, modified heparins and other GAGs are being developed that retain the anti-elastase and anti-inflammatory qualities of heparin with minimal to no anticoagulant activity. The modified heparin, 2-O, 3-O desulfated heparin (ODSH), maintains anti-elastase and anti-inflammatory activities in vitro and in vivo, and has little residual anticoagulant activity. Heparan sulfate with O-sulfate residues but not N-sulfate residues blocks allergic asthmatic inflammation in a murine model. Polysulfated hyaluronic acid abrogates allergen- triggered rhinosinusitis in a murine model. Finally, nonsaccharide glycosaminoglycan mimetics with specific sulfate modifications can be designed to inhibit NE activity. Altogether, these novel GAGs or GAG mimetics hold significant promise to address the unmet need for inhaled anti-elastase and anti-inflammatory therapy for patients with CF.
Keywords: High Mobility Group Box 1; cystic fibrosis; glycosaminoglycans; heparin; hyaluronic acid; neutrophil elastase.
Copyright © 2020 Voynow, Zheng and Kummarapurugu.
Figures

References
-
- Barth P., Bruijnzeel P., Wach A., Sellier Kessler O., Hooftman L., Zimmermann J., et al. (2019). Single dose escalation studies with inhaled POL6014, a potent novel selective reversible inhibitor of human neutrophil elastase, in healthy volunteers and subjects with cystic fibrosis. J. Cyst. Fibros. 19, 299–304. 10.1016/j.jcf.2019.08.020 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

