Approaches to High-Throughput Analysis of Cardiomyocyte Contractility
- PMID: 32733259
- PMCID: PMC7362994
- DOI: 10.3389/fphys.2020.00612
Approaches to High-Throughput Analysis of Cardiomyocyte Contractility
Abstract
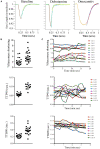
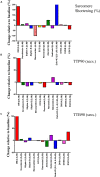
The measurement of the contractile behavior of single cardiomyocytes has made a significant contribution to our understanding of the physiology and pathophysiology of the myocardium. However, the isolation of cardiomyocytes introduces various technical and statistical issues. Traditional video and fluorescence microscopy techniques based around conventional microscopy systems result in low-throughput experimental studies, in which single cells are studied over the course of a pharmacological or physiological intervention. We describe a new approach to these experiments made possible with a new piece of instrumentation, the CytoCypher High-Throughput System (CC-HTS). We can assess the shortening of sarcomeres, cell length, Ca2+ handling, and cellular morphology of almost 4 cells per minute. This increase in productivity means that batch-to-batch variation can be identified as a major source of variability. The speed of acquisition means that sufficient numbers of cells in each preparation can be assessed for multiple conditions reducing these batch effects. We demonstrate the different temporal scales over which the CC-HTS can acquire data. We use statistical analysis methods that compensate for the hierarchical effects of clustering within heart preparations and demonstrate a significant false-positive rate, which is potentially present in conventional studies. We demonstrate a more stringent way to perform these tests. The baseline morphological and functional characteristics of rat, mouse, guinea pig, and human cells are explored. Finally, we show data from concentration response experiments revealing the usefulness of the CC-HTS in such studies. We specifically focus on the effects of agents that directly or indirectly affect the activity of the motor proteins involved in the production of cardiomyocyte contraction. A variety of myocardial preparations with differing levels of complexity are in use (e.g., isolated muscle bundles, thin slices, perfused dual innervated isolated heart, and perfused ventricular wedge). All suffer from low throughput but can be regarded as providing independent data points in contrast to the clustering problems associated with isolated cell studies. The greater productivity and sampling power provided by CC-HTS may help to reestablish the utility of isolated cell studies, while preserving the unique insights provided by studying the contribution of the fundamental, cellular unit of myocardial contractility.
Keywords: calcium; cardiomyocytes; contractility; high-throughput; sarcomere.
Copyright © 2020 Wright, Tsui, Francis, MacLeod and Marston.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

