Metabolic Flexibility and Innate Immunity in Renal Ischemia Reperfusion Injury: The Fine Balance Between Adaptive Repair and Tissue Degeneration
- PMID: 32733450
- PMCID: PMC7358591
- DOI: 10.3389/fimmu.2020.01346
Metabolic Flexibility and Innate Immunity in Renal Ischemia Reperfusion Injury: The Fine Balance Between Adaptive Repair and Tissue Degeneration
Abstract
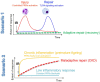
Renal ischemia reperfusion injury (IRI), a common event after renal transplantation, causes acute kidney injury (AKI), increases the risk of delayed graft function (DGF), primes the donor kidney for rejection, and contributes to the long-term risk of graft loss. In the last decade, epidemiological studies have linked even mild episodes of AKI to chronic kidney disease (CKD) progression, and innate immunity seems to play a crucial role. The ischemic insult triggers an acute inflammatory reaction that is elicited by Pattern Recognition Receptors (PRRs), expressed on both infiltrating immune cells as well as tubular epithelial cells (TECs). Among the PRRs, Toll-like receptors (TLRs), their synergistic receptors, Nod-like receptors (NLRs), and the inflammasomes, play a pivotal role in shaping inflammation and TEC repair, in response to renal IRI. These receptors represent promising targets to modulate the extent of inflammation, but also function as gatekeepers of tissue repair, protecting against AKI-to-CKD progression. Despite the important considerations on timely use of therapeutics, in the context of IRI, treatment options are limited by a lack of understanding of the intra- and intercellular mechanisms associated with the activation of innate immune receptors and their impact on adaptive tubular repair. Accumulating evidence suggests that TEC-associated innate immunity shapes the tubular response to stress through the regulation of immunometabolism. Engagement of innate immune receptors provides TECs with the metabolic flexibility necessary for their plasticity during injury and repair. This could significantly affect pathogenic processes within TECs, such as cell death, mitochondrial damage, senescence, and pro-fibrotic cytokine secretion, well-known to exacerbate inflammation and fibrosis. This article provides an overview of the past 5 years of research on the role of innate immunity in experimental and human IRI, with a focus on the cascade of events activated by hypoxic damage in TECs: from programmed cell death (PCD) and mitochondrial dysfunction-mediated metabolic rewiring of TECs to maladaptive repair and progression to fibrosis. Finally, we will discuss the important crosstalk between metabolism and innate immunity observed in TECs and their therapeutic potential in both experimental and clinical research.
Keywords: cell death; innate immunity; kidney transplantation; mitochondria; senescence; tubular repair.
Copyright © 2020 Tammaro, Kers, Scantlebery and Florquin.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

