Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis
- PMID: 32733460
- PMCID: PMC7358580
- DOI: 10.3389/fimmu.2020.01390
Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis
Abstract
Background: Butyric acid (BA) is a short-chain fatty acid (SCFA) with anti-inflammatory properties, which promotes intestinal barrier function. Medium-chain fatty acids (MCFA), including caproic acid (CA), promote TH1 and TH17 differentiation, thus supporting inflammation. Aim: Since most SCFAs are absorbed in the cecum and colon, the measurement of BA in peripheral blood could provide information on the health status of the intestinal ecosystem. Additionally, given the different immunomodulatory properties of BA and CA the evaluation of their serum concentration, as well as their ratio could be as a simple and rapid biomarker of disease activity and/or treatment efficacy in MS. Methods: We evaluated serum BA and CA concentrations, immune parameters, intestinal barrier integrity and the gut microbiota composition in patients with multiple sclerosis (MS) comparing result to those obtained in healthy controls. Results: In MS, the concentration of BA was reduced and that of CA was increased. Concurrently, the microbiota was depleted of BA producers while it was enriched in mucin-degrading, pro-inflammatory components. The reduced serum concentration of BA seen in MS patients correlated with alterations of the barrier permeability, as evidenced by the higher plasma concentrations of lipopolysaccharide and intestinal fatty acid-binding protein, and inflammation. Specifically, CA was positively associated with CD4+/IFNγ+ T lymphocytes, and the BA/CA ratio correlated positively with CD4+/CD25high/Foxp3+ and negatively with CD4+/IFNγ+ T lymphocytes. Conclusion: The gut microbiota dysbiosis found in MS is possibly associated with alterations of the SCFA/MCFA ratio and of the intestinal barrier; this could explain the chronic inflammation that characterizes this disease. SCFA and MCFA quantification could be a simple biomarker to evaluate the efficacy of therapeutic and rehabilitation procedures in MS.
Keywords: T lymphocytes; butyric acid; caproic acid; cytokines; dysbiosis; gut microbiota; multiple sclerosis; short-chain fatty acids (SCFAs).
Copyright © 2020 Saresella, Marventano, Barone, La Rosa, Piancone, Mendozzi, d'Arma, Rossi, Pugnetti, Roda, Casagni, Cas, Paroni, Brigidi, Turroni and Clerici.
Figures


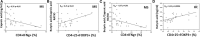




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

