Characterization of a Novel Compound That Stimulates STING-Mediated Innate Immune Activity in an Allele-Specific Manner
- PMID: 32733475
- PMCID: PMC7360819
- DOI: 10.3389/fimmu.2020.01430
Characterization of a Novel Compound That Stimulates STING-Mediated Innate Immune Activity in an Allele-Specific Manner
Abstract
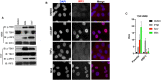
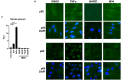
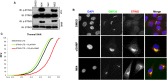
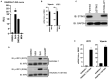
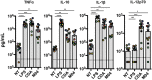
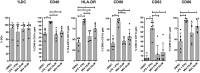
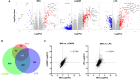
The innate immune response to cytosolic DNA involves transcriptional activation of type I interferons (IFN-I) and proinflammatory cytokines. This represents the culmination of intracellular signaling pathways that are initiated by pattern recognition receptors that engage DNA and require the adaptor protein Stimulator of Interferon Genes (STING). These responses lead to the generation of cellular and tissue states that impair microbial replication and facilitate the establishment of long-lived, antigen-specific adaptive immunity. Ultimately this can lead to immune-mediated protection from infection but also to the cytotoxic T cell-mediated clearance of tumor cells. Intriguingly, pharmacologic activation of STING-dependent phenotypes is known to enhance both vaccine-associated immunogenicity and immune-based anti-tumor therapies. Unfortunately, the STING protein exists as multiple variant forms in the human population that exhibit differences in their reactivity to chemical stimuli and in the intensity of molecular signaling they induce. In light of this, STING-targeting drug discovery efforts require an accounting of protein variant-specific activity. Herein we describe a small molecule termed M04 that behaves as a novel agonist of human STING. Importantly, we find that the molecule exhibits a differential ability to activate STING based on the allelic variant examined. Furthermore, while M04 is inactive in mice, expression of human STING in mouse cells rescues reactivity to the compound. Using primary human cells in ex vivo assays we were also able to show that M04 is capable of simulating innate responses important for adaptive immune activation such as cytokine secretion, dendritic cell maturation, and T cell cross-priming. Collectively, this work demonstrates the conceivable utility of a novel agonist of human STING both as a research tool for exploring STING biology and as an immune potentiating molecule.
Keywords: STING; adjuvant; cytokine; cytosolic DNA sensing; interferon.
Copyright © 2020 Abraham, Botto, Mizuno, Pryke, Gall, Boehm, Sali, Jin, Nilsen, Gough, Baird, Chakhtoura, Subra, Trautmann, Haddad and DeFilippis.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

