High Expression of FAP in Colorectal Cancer Is Associated With Angiogenesis and Immunoregulation Processes
- PMID: 32733792
- PMCID: PMC7362758
- DOI: 10.3389/fonc.2020.00979
High Expression of FAP in Colorectal Cancer Is Associated With Angiogenesis and Immunoregulation Processes
Abstract
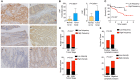
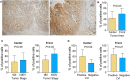
Fibroblast activation protein α (FAP) plays an important role in tissue remodeling and helps tumor cells invade surrounding tissue. We sought to investigate FAP as a prognostic molecular marker in colorectal cancer (CRC) using immunohistochemical and transcriptomic data. FAP expression and clinicopathological information were obtained from The Cancer Genome Atlas data set. The association of FAP expression and tissue cellular heterogeneity landscape was explored using the xCell method. We evaluated FAP protein expression in a cohort of 92 CRCs and 19 non-tumoral tissues. We observed that FAP was upregulated in tumors both at the mRNA and protein levels, and its expression was associated with advanced stages, poor survival, and consensus molecular subtype 4. FAP expression was also associated with angiogenesis and collagen degradation. We observed an enrichment in immune-cell process-related genes associated with FAP overexpression. Colorectal cancers with high FAP expression display an inflamed phenotype enriched for macrophages and monocytes. Those tumors showed enrichment for regulatory T cell populations and depletion of TH1 and natural killer T cells, pointing to an immunosuppressive environment. Colorectal cancers with high levels of stromal FAP are associated with aggressive disease progression and survival. Our results suggest that FAP plays additional roles in tumor progression such as modulation of angiogenesis and immunoregulation in the tumor microenvironment.
Keywords: FAP; cancer-associated fibroblast; colorectal cancer; gene expression; immunohistochemistry.
Copyright © 2020 Coto-Llerena, Ercan, Kancherla, Taha-Mehlitz, Eppenberger-Castori, Soysal, Ng, Bolli, von Flüe, Nicolas, Terracciano, Fani and Piscuoglio.
Figures


References
-
- Eriksen AC, Sørensen FB, Lindebjerg J, Hager H, Christensen RD, Kjær-Frifeldt S, et al. The prognostic value of tumour stroma ratio and tumour budding in stage II colon cancer. A nationwide population-based study Int J Colorectal Dis. (2018) 33:1115–24. 10.1007/s00384-018-3076-9 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

