Angustoline Inhibited Esophageal Tumors Through Regulating LKB1/AMPK/ELAVL1/LPACT2 Pathway and Phospholipid Remodeling
- PMID: 32733803
- PMCID: PMC7358378
- DOI: 10.3389/fonc.2020.01094
Angustoline Inhibited Esophageal Tumors Through Regulating LKB1/AMPK/ELAVL1/LPACT2 Pathway and Phospholipid Remodeling
Abstract
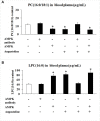
Esophageal cancer is a type of gastrointestinal carcinoma and is among the 10 most common causes of cancer death worldwide. However, the specific mechanism and the biomarkers in the proliferation and metastasis of esophageal tumors are still unclear. Therefore, the development of several natural products which could inhibit esophageal tumors deserve attention. In the present study, different sources of cancer cells were used to select the sensitive cell line (esophageal cancer cell KYSE450) and the proper dose of angustoline, which were utilized in the following cell viability, migration and invasion assays. Then the lipidomic detection of clinical samples (tissue and blood plasma) from esophageal cancer patients was performed, to screen out the specific phospholipid metabolites [PC (16:0/18:1) and LPC (16:0)]. Considering lysophosphatidylcholine acyltransferase 2 (LPCAT2) was tightly relative with phospholipids conversion, serine/threonine-protein kinase 11 (LKB1), 5'-monophosphate (AMP)-activated protein kinase (AMPK) and embryonic lethal, and abnormal vision, drosophila-like 1 (ELAVL1) were investigated, to evaluate their expression levels in esophageal tumor tissue and KYSE450 cells. Additionally, KYSE450 tumor bearing mouse model was constructed, the role of angustoline in inhibiting esophageal tumors through regulating LKB1/AMPK/ELAVL1/LPCAT2 pathway was validated, and found that the conversion from LPC (16:0) to PC (16:0/18:1) was blocked by angustoline in some degree. The above results for the first time proved that angustoline suppressed esophageal tumors through activating LKB1/AMPK and inhibiting ELAVL1/LPCAT2, which consequently blocked phospholipid remodeling from LPC (16:0) to PC (16:0/18:1).
Keywords: AMPK; ELAVL1; LKB1; LPCAT2; esophageal cancer; lipidomics.
Copyright © 2020 Li, Zhang, Zhang, Yao, Yang, Fan and Zheng.
Figures





References
-
- Wang L, Liu X, Yang Q. Application of metabolomics in cancer research: as a powerful tool to screen biomarker for diagnosis, monitoring and prognosis of cancer. Biomarkers J. (2018) 4:12 10.21767/2472-1646.100050 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

