Evaluation of a tumor electric field treatment system in a rat model of glioma
- PMID: 32734621
- PMCID: PMC7564191
- DOI: 10.1111/cns.13441
Evaluation of a tumor electric field treatment system in a rat model of glioma
Abstract
Objective: Glioma is a devastating disease lacking effective treatment. Tumor electric field therapy is emerging as a novel non-invasive therapy. The current study evaluates the efficacy and safety of a self-designed tumor electric field therapy system (TEFTS ASCLU-300) in a rat orthotopic transplantation model of glioma.
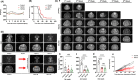
Methods: A model of intracranial orthotopic transplantation was established in rats using glioma C6 cells. For electric field therapy, glioma-bearing rats were exposed to alternating electric fields generated by a self-developed TEFTS starting on either 1st (Group 2) or 3rd (Group 3) day after transplantation, while other conditions were maintained the same as non-treated rats (Group 1). Glioma size, body weight, and overall survival (OS) were compared between groups. Immunohistochemical staining was applied to access tumor cell death and microvessel density within the tumor. In addition, the systemic effects of TEFTS on blood cells, vital organs, and hepatorenal functions were evaluated.
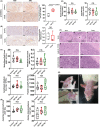
Results: TEFTS treatment significantly elongated the OS of tumor-bearing rats compared with non-treated rats (non-treated vs treated: 24.77 ± 7.08 days vs 40.31 ± 19.11 days, P = .0031). Continuous TEFTS treatment starting on 1st or 3rd day significantly reduced glioma size at 2 and 3 weeks after tumor cell inoculation (Week 2: Group 1:289.95 ± 101.69 mm3 ; Group 2:70.45 ± 17.79 mm3 ; Group 3:73.88 ± 33.21 mm3 , P < .0001. Week 3: Group 1:544.096 ± 78.53 mm3 ; Group 2:187.58 ± 78.44 mm3 ; Group 3:167.14 ± 109.96 mm3 , P = .0005). Continuous treatment for more than 4 weeks inhibited tumor growth. The TEFTS treatment promoted tumor cell death, as demonstrated by increased number of Caspase 3+ cells within the tumor (non-treated vs treated: 38.06 ± 10.04 vs 68.57 ± 8.09 cells/field, P = .0007), but had minimal effect on microvessel density, as shown by CD31 expression (non-treated vs treated: 1.63 ± 0.09 vs 1.57 ± 0.13% of positively stained areas, P > .05). No remarkable differences were observed in hepatorenal function, blood cell counts, or other vital organs between non-treated and treated groups.
Conclusion: The TEFTS developed by our research team was proved to be effective and safe to inhibit tumor growth and improve general outcomes in a rat model of brain glioma.
Keywords: cell death; electric field therapy; glioma; survival; tumor electric field treatment system; tumor size.
© 2020 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wenger C, Miranda PC, Salvador R, et al. A review on tumor‐treating fields (TTFields): clinical implications inferred from computational modeling. IEEE Rev Biomed Eng. 2018;11:195‐207. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

