Drug Resistance Mutations Among South African Children Living With HIV on WHO-recommended ART Regimens
- PMID: 32735012
- PMCID: PMC11657150
- DOI: 10.1093/cid/ciaa1068
Drug Resistance Mutations Among South African Children Living With HIV on WHO-recommended ART Regimens
Abstract
Background: Children living with human immunodeficiency virus (HIV) (CLHIV) receiving antiretroviral therapy (ART) in resource-limited settings are susceptible to high rates of acquired HIV drug resistance (HIVDR), but few studies include children initiating age-appropriate World Health Organization (WHO)-recommended first-line regimens. We report data from a cohort of ART-naive South African children who initiated first-line ART.
Methods: ART-eligible CLHIV aged 0-12 years were enrolled from 2012 to 2014 at 5 public South African facilities and were followed for up to 24 months. Enrolled CLHIV received standard-of-care WHO-recommended first-line ART. At the final study visit, a dried blood spot sample was obtained for viral load and genotypic resistance testing.
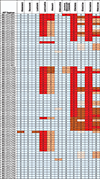
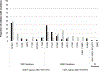
Results: Among 72 successfully genotyped CLHIV, 49 (68.1%) received ABC/3TC/LPV/r, and 23 (31.9%) received ABC/3TC/EFV. All but 2 children on ABC/3TC/LPV/r were <3 years, and all CLHIV on ABC/3TC/EFV were ≥3 years. Overall, 80.6% (58/72) had at least one drug resistance mutation (DRM). DRMs to nonnucleoside reverse transcriptase inhibitors (NNRTIs) and nucleoside reverse transcriptase inhibitors (NRTIs) were found among 65% and 51% of all CLHIV, respectively, with no statistical difference by ART regimen. More CLHIV on ABC/3TC/EFV, 47.8% (11/23), were found to have 0 or only 1 effective antiretroviral drug remaining in their current regimen compared to 8.2% (4/49) on ABC/3TC/LPV/r.
Conclusions: High levels of NNRTI and NRTI DRMs among CLHIV receiving ABC/3TC/LPV/r suggests a lasting impact of failed mother-to-child transmission interventions on DRMs. However, drug susceptibility analysis reveals that CLHIV with detectable viremia on ABC/3TC/LPV/r are more likely to have maintained at least 2 effective agents on their current HIV regimen than those on ABC/3TC/EFV.
Keywords: HIV drug resistance; children; pediatric HIV; resource-limited settings.
Published by Oxford University Press for the Infectious Diseases Society of America 2020.
Conflict of interest statement
Figures

References
-
- UNAIDS. Fact sheet—global AIDS update 2019. Geneva: UNAIDS, 2019.
-
- UNAIDS. Miles to go: closing gaps, breaking barriers, righting injustices. Geneva: UNAIDS, 2018.
-
- Boerma RS, Boender TS, Bussink AP, et al. Suboptimal viral suppression rates among HIV-infected children in low- and middle-income countries: a meta-analysis. Clin Infect Dis 2016; 63:1645–54. - PubMed
-
- Saito S Population HIV impact assessments: what we can learn about pediatric HIV?. In: International Workshop on HIV Pediatrics. Mexico City, Mexico, 2019.
-
- Eswatini GotKo. Swaziland HIV Incidence Measurement Survey 2 (SHIMS2) 2016–2017. Mbabane: Government of the Kingdom of Eswatini, 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

