Unconventional Biocatalytic Approaches to the Synthesis of Chiral Sulfoxides
- PMID: 32735057
- PMCID: PMC7891444
- DOI: 10.1002/cbic.202000430
Unconventional Biocatalytic Approaches to the Synthesis of Chiral Sulfoxides
Abstract
Sulfoxides are a class of organic compounds that find wide application in medicinal and organic chemistry. Several biocatalytic approaches have been developed to synthesise enantioenriched sulfoxides, mainly by exploiting oxidative enzymes. Recently, the use of reductive enzymes such as Msr and Dms has emerged as a new, alternative method to obtain enantiopure sulfoxides from racemic mixtures. In parallel, novel oxidative approaches, employing nonclassical solvents such as ionic liquids (ILs) and deep eutectic solvents (DESs), have been developed as greener and more sustainable biocatalytic synthetic pathways. This minireview aims highlights the recent advances made in the biocatalytic synthesis of enantioenriched sulfoxides by employing such unconventional approaches.
Keywords: biocatalysis; deep eutectics; ionic liquids; reductive enzymes; sulfoxides.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

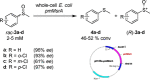
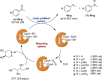



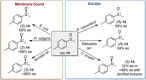
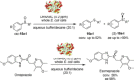
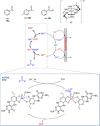
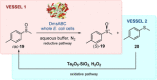


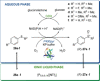
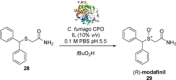
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

