Effectiveness of a Mobile App Intervention for Anxiety and Depression Symptoms in University Students: Randomized Controlled Trial
- PMID: 32735221
- PMCID: PMC7428915
- DOI: 10.2196/15418
Effectiveness of a Mobile App Intervention for Anxiety and Depression Symptoms in University Students: Randomized Controlled Trial
Abstract
Background: Depression and anxiety symptoms are common among university students, but many do not receive treatment. This is often because of lack of availability, reluctance to seek help, and not meeting the diagnostic criteria required to access services. Internet-based interventions, including smartphone apps, can overcome these issues. However, a large number of apps are available, each with little evidence of their effectiveness.
Objective: This study aims to evaluate for the first time the effectiveness of a self-guided mobile app, Feel Stress Free, for the treatment of depression and anxiety symptoms in students.
Methods: A web-based randomized controlled trial compared a cognitive behavioral therapy (CBT)-based mobile app Feel Stress Free with a wait-list control. University students self-identified as experiencing symptoms of anxiety or depression and were randomized to 6 weeks of intervention (n=84) or control (n=84), unblinded. The app is self-guided and incorporates behavioral relaxation activities, mood tracking and thought challenging, and minigames. Participants completed the Hospital Anxiety and Depression Scale online at baseline and every fortnight.
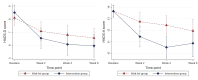
Results: At week 6, the primary end point, there was evidence that the Feel Stress Free app reduced depression symptoms (mean difference -1.56; 95% CI -2.67 to -0.44; P=.006) but only very weak evidence that it reduced anxiety symptoms (mean difference -1.36; 95% CI -2.93 to 0.21; P=.09). At week 4, there was evidence to support the effectiveness of the intervention for anxiety symptoms (mean difference -1.94; 95% CI -3.11 to -0.77; P=.001) and, though weaker, depression symptoms (mean difference -1.08; 95% CI -2.12 to -0.04; P=.04). At week 6, 83% (34/41) of participants indicated that they were using the app weekly or more frequently.
Conclusions: The Feel Stress Free app is a promising mobile intervention for treating symptoms of anxiety and depression in students and overcomes many of the barriers to traditional CBT. Further research is needed to establish its effectiveness at and beyond 6 weeks.
Trial registration: ClinicalTrials.gov NCT03032952; https://clinicaltrials.gov/ct2/show/NCT03032952.
Keywords: anxiety; cognitive behavioral therapy; depression; eHealth; mobile apps; mobile phone; online intervention; randomized controlled trial.
©Tayla McCloud, Rebecca Jones, Gemma Lewis, Vaughan Bell, Elias Tsakanikos. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 31.07.2020.
Conflict of interest statement
Conflicts of Interest: Dr Andres Fonseca is a consultant psychiatrist and Co-Founder of Thrive Therapeutic Software Ltd, the company that developed the app. This trial was designed in collaboration with Dr Fonseca, and Thrive provided the app to participants for free, assisted with technical difficulties and contacting universities, and covered recruitment costs. Preparation of this manuscript was completed by the authors independently of Thrive, and Thrive did not have any involvement in review or approval of the final manuscript. The authors report no other conflicts of interest.
Figures

References
-
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey Replication The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R) J Am Med Assoc. 2003 Jun 18;289(23):3095–105. doi: 10.1001/jama.289.23.3095. - DOI - PubMed
-
- Kessler RC, Angermeyer M, Anthony JC, de Graaf R, Demyttenaere K, Gasquet I, de Girolamo G, Gluzman S, Gureje O, Haro JM, Kawakami N, Karam A, Levinson D, Medina Mora ME, Browne MA, Posada-Villa J, Stein DJ, Tsang CH, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell BE, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustün TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the world health organization's world mental health survey initiative. World Psychiatry. 2007 Oct;6(3):168–76. https://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pu... - PMC - PubMed
-
- Thorley C. Not By Degrees: Improving Student Mental Health in the UK's Universities. IPPR. 2017. [2020-06-16]. http://www.ippr.org/research/publications/not-by-degrees.

