Zinc protection of fertilized eggs is an ancient feature of sexual reproduction in animals
- PMID: 32735558
- PMCID: PMC7423145
- DOI: 10.1371/journal.pbio.3000811
Zinc protection of fertilized eggs is an ancient feature of sexual reproduction in animals
Abstract
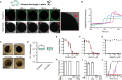
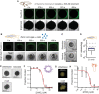
One of the earliest and most prevalent barriers to successful reproduction is polyspermy, or fertilization of an egg by multiple sperm. To prevent these supernumerary fertilizations, eggs have evolved multiple mechanisms. It has recently been proposed that zinc released by mammalian eggs at fertilization may block additional sperm from entering. Here, we demonstrate that eggs from amphibia and teleost fish also release zinc. Using Xenopus laevis as a model, we document that zinc reversibly blocks fertilization. Finally, we demonstrate that extracellular zinc similarly disrupts early embryonic development in eggs from diverse phyla, including Cnidaria, Echinodermata, and Chordata. Our study reveals that a fundamental strategy protecting human eggs from fertilization by multiple sperm may have evolved more than 650 million years ago.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

