Delayed short-term tamoxifen treatment does not promote remyelination or neuron sparing after spinal cord injury
- PMID: 32735618
- PMCID: PMC7394399
- DOI: 10.1371/journal.pone.0235232
Delayed short-term tamoxifen treatment does not promote remyelination or neuron sparing after spinal cord injury
Abstract
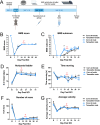
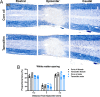
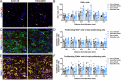
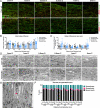
The tamoxifen-dependent Cre/lox system in transgenic mice has become an important research tool across all scientific disciplines for manipulating gene expression in specific cell types. In these mouse models, Cre-recombination is not induced until tamoxifen is administered, which allows researchers to have temporal control of genetic modifications. Interestingly, tamoxifen has been identified as a potential therapy for spinal cord injury (SCI) and traumatic brain injury patients due to its neuroprotective properties. It is also reparative in that it stimulates oligodendrocyte differentiation and remyelination after toxin-induced demyelination. However, it is unknown whether tamoxifen is neuroprotective and neuroreparative when administration is delayed after SCI. To properly interpret data from transgenic mice in which tamoxifen treatment is delayed after SCI, it is necessary to identify the effects of tamoxifen alone on anatomical and functional recovery. In this study, female and male mice received a moderate mid-thoracic spinal cord contusion. Mice were then gavaged with corn oil or a high dose of tamoxifen from 19-22 days post-injury, and sacrificed 42 days post-injury. All mice underwent behavioral testing for the duration of the study, which revealed that tamoxifen treatment did not impact hindlimb motor recovery. Similarly, histological analyses revealed that tamoxifen had no effect on white matter sparing, total axon number, axon sprouting, glial reactivity, cell proliferation, oligodendrocyte number, or myelination, but tamoxifen did decrease the number of neurons in the dorsal and ventral horn. Semi-thin sections confirmed that axon demyelination and remyelination were unaffected by tamoxifen. Sex-specific responses to tamoxifen were also assessed, and there were no significant differences between female and male mice. These data suggest that delayed tamoxifen administration after SCI does not change functional recovery or improve tissue sparing in female or male mice.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Continuous tamoxifen delivery improves locomotor recovery 6h after spinal cord injury by neuronal and glial mechanisms in male rats.Exp Neurol. 2018 Jan;299(Pt A):109-121. doi: 10.1016/j.expneurol.2017.10.006. Epub 2017 Oct 13. Exp Neurol. 2018. PMID: 29037533 Free PMC article.
-
Therapeutic administration of mouse mast cell protease 6 improves functional recovery after traumatic spinal cord injury in mice by promoting remyelination and reducing glial scar formation.FASEB J. 2023 Jun;37(6):e22939. doi: 10.1096/fj.202201942RR. FASEB J. 2023. PMID: 37130013
-
Decellularized extracellular matrix enriched with GDNF enhances neurogenesis and remyelination for improved motor recovery after spinal cord injury.Acta Biomater. 2024 May;180:308-322. doi: 10.1016/j.actbio.2024.04.015. Epub 2024 Apr 16. Acta Biomater. 2024. PMID: 38615813
-
Remyelination after spinal cord injury: is it a target for repair?Prog Neurobiol. 2014 Jun;117:54-72. doi: 10.1016/j.pneurobio.2014.02.006. Epub 2014 Feb 28. Prog Neurobiol. 2014. PMID: 24582777 Review.
-
Oligodendrogliogenesis and Axon Remyelination after Traumatic Spinal Cord Injuries in Animal Studies: A Systematic Review.Neuroscience. 2019 Mar 15;402:37-50. doi: 10.1016/j.neuroscience.2019.01.019. Epub 2019 Jan 24. Neuroscience. 2019. PMID: 30685542
Cited by
-
Chronic demyelination and myelin repair after spinal cord injury in mice: A potential link for glutamatergic axon activity.Glia. 2023 Sep;71(9):2096-2116. doi: 10.1002/glia.24382. Epub 2023 May 20. Glia. 2023. PMID: 37208933 Free PMC article.
-
Spinal cord injury-induced metabolic impairment and steatohepatitis develops in non-obese rats and is exacerbated by premorbid obesity.Exp Neurol. 2024 Sep;379:114847. doi: 10.1016/j.expneurol.2024.114847. Epub 2024 Jun 8. Exp Neurol. 2024. PMID: 38852834 Free PMC article.
-
Data reporting quality and semantic interoperability increase with community-based data elements (CoDEs). Analysis of the open data commons for spinal cord injury (ODC-SCI).Exp Neurol. 2025 Mar;385:115100. doi: 10.1016/j.expneurol.2024.115100. Epub 2024 Dec 7. Exp Neurol. 2025. PMID: 39647573 Free PMC article.
-
Deficiency of galactosyl-ceramidase in adult oligodendrocytes worsens disease severity during chronic experimental allergic encephalomyelitis.Mol Ther. 2024 Sep 4;32(9):3163-3176. doi: 10.1016/j.ymthe.2024.06.035. Epub 2024 Jun 26. Mol Ther. 2024. PMID: 38937968 Free PMC article.
-
Paclitaxel Chemotherapy Elicits Widespread Brain Anisotropy Changes in a Comprehensive Mouse Model of Breast Cancer Survivorship: Evidence From In Vivo Diffusion Weighted Imaging.Front Oncol. 2022 Mar 23;12:798704. doi: 10.3389/fonc.2022.798704. eCollection 2022. Front Oncol. 2022. PMID: 35402248 Free PMC article.
References
-
- National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. Birmingham, AL; 2019.
-
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006. December;129(12):3249–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

