IL-15 Preconditioning Augments CAR T Cell Responses to Checkpoint Blockade for Improved Treatment of Solid Tumors
- PMID: 32735774
- PMCID: PMC7647667
- DOI: 10.1016/j.ymthe.2020.07.018
IL-15 Preconditioning Augments CAR T Cell Responses to Checkpoint Blockade for Improved Treatment of Solid Tumors
Abstract
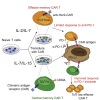
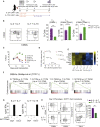
Chimeric antigen receptor (CAR) T cell therapy has been highly successful in hematological malignancies leading to their US Food and Drug Administration (FDA) approval. However, the efficacy of CAR T cells in solid tumors is limited by tumor-induced immunosuppression, leading to the development of combination approaches, such as adjuvant programmed cell death 1 (PD-1) blockade. Current FDA-approved methods for generating CAR T cells utilize either anti-CD3 and interleukin (IL)-2 or anti-CD3/CD28 beads, which can generate a T cell product with an effector/exhausted phenotype. Whereas different cytokine preconditioning milieu, such as IL-7/IL-15, have been shown to promote T cell engraftment, the impact of this approach on CAR T cell responses to adjuvant immune-checkpoint blockade has not been assessed. In the current study, we reveal that the preconditioning of CAR T cells with IL-7/IL-15 increased CAR T cell responses to anti-PD-1 adjuvant therapy. This was associated with the emergence of an intratumoral CD8+CD62L+TCF7+IRF4- population that was highly responsive to anti-PD-1 therapy and mediated the vast majority of transcriptional and epigenetic changes in vivo following PD-1 blockade. Our data indicate that preservation of CAR T cells in a TCF7+ phenotype is crucial for their responsiveness to adjuvant immunotherapy approaches and should be a key consideration when designing clinical protocols.
Keywords: CAR T cells; IL-15; T(CM); TCF1; TCF7; anti-PD-1; cancer; checkpoint blockade; solid tumor.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Carpenito C., Milone M.C., Hassan R., Simonet J.C., Lakhal M., Suhoski M.M., Varela-Rohena A., Haines K.M., Heitjan D.F., Albelda S.M. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. USA. 2009;106:3360–3365. - PMC - PubMed
-
- Finney H.M., Lawson A.D., Bebbington C.R., Weir A.N. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 1998;161:2791–2797. - PubMed
-
- Mardiana S., Solomon B.J., Darcy P.K., Beavis P.A. Supercharging adoptive T cell therapy to overcome solid tumor-induced immunosuppression. Sci. Transl. Med. 2019;11:eaaw2293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

