Key role of estrogen receptor β in the organization of brain and behavior of the Japanese quail
- PMID: 32735801
- PMCID: PMC7541764
- DOI: 10.1016/j.yhbeh.2020.104827
Key role of estrogen receptor β in the organization of brain and behavior of the Japanese quail
Abstract
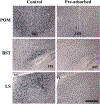
Estrogens play a key role in the sexual differentiation of the brain and behavior. While early estrogen actions exert masculinizing effects on the brain of male rodents, a diametrically opposite effect is observed in birds where estrogens demasculinize the brain of females. Yet, the two vertebrate classes express similar sex differences in the brain and behavior. Although ERα is thought to play a major role in these processes in rodents, the role of ERβ is still controversial. In birds, the identity of the estrogen receptor(s) underlying the demasculinization of the female brain remains unclear. The aim of the present study was thus to determine in Japanese quail the effects of specific agonists of ERα (propylpyrazole triol, PPT) and ERβ (diarylpropionitrile, DPN) administered at the beginning of the sensitive period (embryonic day 7, E7) on the sexual differentiation of male sexual behavior and on the density of vasotocin-immunoreactive (VT-ir) fibers, a known marker of the organizational action of estrogens on the quail brain. We demonstrate that estradiol benzoate and the ERβ agonist (DPN) demasculinize male sexual behavior and decrease the density of VT-ir fibers in the medial preoptic nucleus and the bed nucleus of the stria terminalis, while PPT has no effect on these measures. These results clearly indicate that ERβ, but not ERα, is involved in the estrogen-induced sexual differentiation of brain and sexual behavior in quail.
Keywords: DPN; Estrogen receptor α; Estrogen receptor β; PPT; Sexual differentiation; Vasotocin.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures

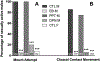



References
-
- Adkins-Regan E, Pickett P, & Koutnik D (1982). Sexual differentiation in quail: Conversion of androgen to estrogen mediates testosterone-induced demasculinization of copulation but not other male characteristics. Hormones and Behavior, 16(3), 259–278. doi: 10.1016/0018-506X(82)90026-5 - DOI - PubMed
-
- Adkins EK (1975). Hormonal basis of sexual differentiation in the Japanese quail. Journal of comparative and physiological psychology, 89(1), 61. - PubMed
-
- Adkins EK (1978). Sex Steroids and the Differentiation of Avian Reproductive Behavior. American Zoologist, 18(3), 501–509. doi: 10.1093/icb/18.3.501 - DOI
-
- Adkins EK, & Adler NT (1972). Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol, 81(1), 27–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

