Nanofibrous spongy microspheres to deliver rabbit mesenchymal stem cells and anti-miR-199a to regenerate nucleus pulposus and prevent calcification
- PMID: 32736170
- PMCID: PMC7423691
- DOI: 10.1016/j.biomaterials.2020.120213
Nanofibrous spongy microspheres to deliver rabbit mesenchymal stem cells and anti-miR-199a to regenerate nucleus pulposus and prevent calcification
Abstract
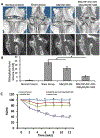
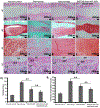
Lower back pain is mainly caused by intervertebral disc degeneration, in which calcification is frequently involved. Here novel nanofibrous spongy microspheres (NF-SMS) are used to carry rabbit bone marrow mesenchymal stromal cells (MSCs) to regenerate nucleus pulposus tissues. NF-SMS are shown to significantly enhance the MSC seeding, proliferation and differentiation over control microcarriers. Furthermore, a hyperbranched polymer (HP) with negligible cytotoxicity and high microRNA (miRNAs) binding affinity is synthesized. The HP can complex with anti-miR-199a and self-assemble into "double shell" polyplexes which are able to achieve high transfection efficiency into MSCs. A double-emulsion technique is used to encapsulate these polyplexes in biodegradable nanospheres (NS) to enable sustained anti-miR-199 delivery. Our results demonstrate that MSC/HP-anti-miR-199a/NS/NF-SMS constructs can promote the nucleus pulposus (NP) phenotype and resist calcification in vitro and in a subcutaneous environment. Furthermore, injection of MSC/HP-anti-miR-199a/NS/NF-SMS can stay in place, produce functional extracellular matrix, maintain disc height and prevent intervertebral disc (IVD) calcification in a rabbit lumbar degeneration model.
Keywords: Anti-microRNA oligonucleotides; Calcification; Mesenchymal stem cells; Nucleus pulposus; Rabbit; Regeneration.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





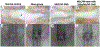
References
-
- Murab S, Samal J, Shrivastava A, Ray AR, Pandit A, Ghosh S, Glucosamine loaded injectable silk-in-silk integrated system modulate mechanical properties in bovine ex-vivo degenerated intervertebral disc model, Biomaterials 55 (2015) 64–83. - PubMed
-
- Schmocker A, Khoushabi A, Frauchiger DA, Gantenbein B, Schizas C, Moser C, Bourban PE, Pioletti DP, A photopolymerized composite hydrogel and surgical implanting tool for a nucleus pulposus replacement, Biomaterials 88 (2016) 110–9. - PubMed
-
- Li Y, Samartzis D, Campbell D, Cherny S, Cheung KM, Luk KD, Karppinen J, Song Y, Cheah K, Chan D, Sham PC, Two subtypes of intervertebral disc degeneration distinguished by large-scale population-based study, Spine J (2016). - PubMed
-
- Boos N, Nerlich AG, Wiest I, von der Mark K, Aebi M, Immunolocalization of type X collagen in human lumbar intervertebral discs during ageing and degeneration, Histochem Cell Biol 108(6) (1997) 471–80. - PubMed
-
- Feinberg J, Boachie-Adjei O, Bullough PG, Boskey AL, The distribution of calcific deposits in intervertebral discs of the lumbosacral spine, Clin Orthop Relat Res (254) (1990) 303–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

