A nephrotoxicity-free, iron-based contrast agent for magnetic resonance imaging of tumors
- PMID: 32736259
- PMCID: PMC7442595
- DOI: 10.1016/j.biomaterials.2020.120234
A nephrotoxicity-free, iron-based contrast agent for magnetic resonance imaging of tumors
Abstract
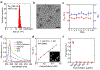
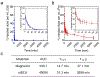
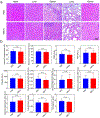
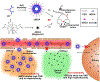
Gadolinium-based contrast agents (GBCAs) are the most widely used T1 contrast agents for magnetic resonance imaging (MRI) and have achieved remarkable success in clinical cancer diagnosis. However, GBCAs could cause severe nephrogenic systemic fibrosis to patients with renal insufficiency. Nevertheless, GBCAs are quickly excreted from the kidneys, which shortens their imaging window and prevents long-term monitoring of the disease per injection. Herein, a nephrotoxicity-free T1 MRI contrast agent is developed by coordinating ferric iron into a telodendritic, micellar nanostructure. This new nano-enabled, iron-based contrast agent (nIBCA) not only can reduce the renal accumulation and relieve the kidney burden, but also exhibit a significantly higher tumor to noise ratio (TNR) for cancer diagnosis. In comparison with Magnevist (a clinical-used GBCA), Magnevist induces obvious nephrotoxicity while nIBCA does not, indicating that such a novel contrast agent may be applicable to renally compromised patients requiring a contrast-enhanced MRI. The nIBCA could precisely image subcutaneous brain tumors in a mouse model and the effective imaging window lasted for at least 24 h. The nIBCA also precisely highlights the intracranial brain tumor with high TNR. The nIBCA presents a potential alternative to GBCAs as it has superior biocompatibility, high TNR and effective imaging window.
Keywords: Contrast agents; Magnetic resonance imaging; Nanoparticle; Nephrotoxicity-free; Tumor imaging.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing interests
The authors declare no competing financial interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Greene FL, Balch CM, Fleming ID, Fritz A, Haller DG, Morrow M, Page DL, AJCC cancer staging handbook: TNM classification of malignant tumors, Springer Science & Business Media, 2002.
-
- Gambhir SS, Molecular imaging of cancer with positron emission tomography, Nat. Rev. Cancer 2(9) (2002) 683–693. - PubMed
-
- Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, von Schulthess GK, Steinert HC, Staging of Non–Small-Cell Lung Cancer with Integrated Positron-Emission Tomography and Computed Tomography, N. Engl. J. Med 348(25) (2003) 2500–2507. - PubMed
-
- Edward Coleman R, Single photon emission computed tomography and positron emission tomography in cancer imaging, Cancer 67(S4) (1991) 1261–1270. - PubMed
-
- Wiele C.V.d., Lahorte C, Vermeersch H, Loose D, Mervillie K, Steinmetz ND, Vanderheyden J-L, Cuvelier CA, Slegers G, Dierck RA, Quantitative Tumor Apoptosis Imaging Using Technetium-99m–HYNIC Annexin V Single Photon Emission Computed Tomography, J. Clin. Oncol 21(18) (2003) 3483–3487. - PubMed

