Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
- PMID: 32736337
- PMCID: PMC7413710
- DOI: 10.1212/NXI.0000000000000817
Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
Abstract
Objective: To determine whether IgG subclasses of antiparanodal autoantibodies are related to disease course and treatment response in acute- to subacute-onset neuropathies, we retrospectively screened 161 baseline serum/CSF samples and 66 follow-up serum/CSF samples.
Methods: We used ELISA and immunofluorescence assays to detect antiparanodal IgG and their subclasses and titers in serum/CSF of patients with Guillain-Barré syndrome (GBS), recurrent GBS (R-GBS), Miller-Fisher syndrome, and acute- to subacute-onset chronic inflammatory demyelinating polyradiculoneuropathy (A-CIDP). We evaluated clinical data retrospectively.
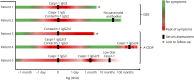
Results: We detected antiparanodal autoantibodies with a prevalence of 4.3% (7/161), more often in A-CIDP (4/23, 17.4%) compared with GBS (3/114, 2.6%). Longitudinal subclass analysis in the patients with GBS revealed IgG2/3 autoantibodies against Caspr-1 and against anti-contactin-1/Caspr-1, which disappeared at remission. At disease onset, patients with A-CIDP had IgG2/3 anti-Caspr-1 and anti-contactin-1/Caspr-1 or IgG4 anti-contactin-1 antibodies, IgG3 being associated with good response to IV immunoglobulins (IVIg). In the chronic phase of disease, IgG subclass of one patient with A-CIDP switched from IgG3 to IgG4.
Conclusion: Our data (1) confirm and extend previous observations that antiparanodal IgG2/3 but not IgG4 antibodies can occur in acute-onset neuropathies manifesting as monophasic GBS, (2) suggest association of IgG3 to a favorable response to IVIg, and (3) lend support to the hypothesis that in some patients, an IgG subclass switch from IgG3 to IgG4 may be the correlate of a secondary progressive or relapsing course following a GBS-like onset.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



Comment in
- Neurol Neuroimmunol Neuroinflamm. 7(5):e843.
References
-
- Fehmi J, Scherer SS, Willison HJ, Rinaldi S. Nodes, paranodes and neuropathies. J Neurol Neurosurg Psychiatry 2018;89:61–71. - PubMed
-
- Uncini A, Vallat JM. Autoimmune nodo-paranodopathies of peripheral nerve: the concept is gaining ground. J Neurol Neurosurg Psychiatry 2018;89:627–635. - PubMed
-
- Querol L, Illa I. Paranodal and other autoantibodies in chronic inflammatory neuropathies. Curr Opin Neurol 2015;28:474–479. - PubMed
-
- Querol L, Rojas-Garcia R, Diaz-Manera J, et al. . Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflamm 2015;2:e149. doi: 10.1212/NXI.0000000000000149. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
