Meta-analysis of mouse transcriptomic studies supports a context-dependent astrocyte reaction in acute CNS injury versus neurodegeneration
- PMID: 32736565
- PMCID: PMC7393869
- DOI: 10.1186/s12974-020-01898-y
Meta-analysis of mouse transcriptomic studies supports a context-dependent astrocyte reaction in acute CNS injury versus neurodegeneration
Abstract
Background: Neuronal damage in acute CNS injuries and chronic neurodegenerative diseases is invariably accompanied by an astrocyte reaction in both mice and humans. However, whether and how the nature of the CNS insult-acute versus chronic-influences the astrocyte response, and whether astrocyte transcriptomic changes in these mouse models faithfully recapitulate the astrocyte reaction in human diseases remains to be elucidated. We hypothesized that astrocytes set off different transcriptomic programs in response to acute versus chronic insults, besides a shared "pan-injury" signature common to both types of conditions, and investigated the presence of these mouse astrocyte signatures in transcriptomic studies from human neurodegenerative diseases.
Methods: We performed a meta-analysis of 15 published astrocyte transcriptomic datasets from mouse models of acute injury (n = 6) and chronic neurodegeneration (n = 9) and identified pan-injury, acute, and chronic signatures, with both upregulated (UP) and downregulated (DOWN) genes. Next, we investigated these signatures in 7 transcriptomic datasets from various human neurodegenerative diseases.
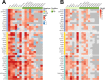
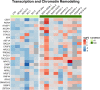
Results: In mouse models, the number of UP/DOWN genes per signature was 64/21 for pan-injury and 109/79 for acute injury, whereas only 13/27 for chronic neurodegeneration. The pan-injury-UP signature was represented by the classic cytoskeletal hallmarks of astrocyte reaction (Gfap and Vim), plus extracellular matrix (i.e., Cd44, Lgals1, Lgals3, Timp1), and immune response (i.e., C3, Serping1, Fas, Stat1, Stat2, Stat3). The acute injury-UP signature was enriched in protein synthesis and degradation (both ubiquitin-proteasome and autophagy systems), intracellular trafficking, and anti-oxidant defense genes, whereas the acute injury-DOWN signature included genes that regulate chromatin structure and transcriptional activity, many of which are transcriptional repressors. The chronic neurodegeneration-UP signature was further enriched in astrocyte-secreted extracellular matrix proteins (Lama4, Cyr61, Thbs4), while the DOWN signature included relevant genes such as Agl (glycogenolysis), S1pr1 (immune modulation), and Sod2 (anti-oxidant). Only the pan-injury-UP mouse signature was clearly present in some human neurodegenerative transcriptomic datasets.
Conclusions: Acute and chronic CNS injuries lead to distinct astrocyte gene expression programs beyond their common astrocyte reaction signature. However, caution should be taken when extrapolating astrocyte transcriptomic findings from mouse models to human diseases.
Keywords: Acute CNS injury; Astrocyte reaction; Meta-analysis; Neurodegenerative diseases; Transcriptomics.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16(5):580–586. - PubMed
-
- Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S, et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [corrected] Cell Stem Cell. 2013;12(4):426–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

