Phase 3 Trial of a Small-volume Subcutaneous 6-Month Duration Leuprolide Acetate Treatment for Central Precocious Puberty
- PMID: 32738042
- PMCID: PMC7442270
- DOI: 10.1210/clinem/dgaa479
Phase 3 Trial of a Small-volume Subcutaneous 6-Month Duration Leuprolide Acetate Treatment for Central Precocious Puberty
Erratum in
-
Corrigendum to: "Phase 3 Trial of a Small-Volume Subcutaneous 6-Month Duration Leuprolide Acetate Treatment for Central Precocious Puberty".J Clin Endocrinol Metab. 2021 Jun 16;106(7):e2842. doi: 10.1210/clinem/dgab294. J Clin Endocrinol Metab. 2021. PMID: 34000029 Free PMC article. No abstract available.
Abstract
Context: Gonadotropin-releasing hormone agonists (GnRHas) are standard of care for central precocious puberty (CPP). A 6-month subcutaneous injection has recently been approved by the Food and Drug Administration.
Objective: Determine efficacy, pharmacokinetics, and safety of 6-month 45-mg subcutaneous leuprolide acetate for CPP.
Design: Phase 3 multicenter, open-label, single-arm study.
Setting: 25 sites in 6 countries.
Subjects: 64 GnRHa-naïve children with CPP (age: 7.5 ± 0.1 years) received study drug: 59 completed the study.
Intervention(s): 2 doses of 45-mg subcutaneous leuprolide acetate (0.375 mL) at 0 and 24 weeks; children were followed for 48 weeks.
Main outcome measure(s): Percentage of children with serum luteinizing hormone (LH) <4 IU/L 30 minutes following GnRHa stimulation at week 24.
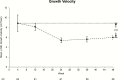
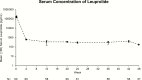
Results: 54/62 (87%) children achieved poststimulation LH <4 IU/L at week 24; 49/56 (88%) girls and 1/2 boys maintained peak LH <4 IU/L at week 48. Mean growth velocity decreased from 8.9 cm/year at week 4 to 6.0 cm/year at week 48. Mean bone age was advanced 3.0 years beyond chronological age at screening and 2.7 years at week 48. Breast pubertal stage regressed or was stable in 97% of girls and external genitalia development regressed in both boys. Adverse events were mild and did not cause treatment discontinuation.
Conclusions: A small volume of 45-mg subcutaneous leuprolide acetate administered at a 6-month interval effectively suppressed pubertal hormones and stopped or caused regression of pubertal progression. This long-acting GnRHa preparation of leuprolide acetate is a new, effective, and well-tolerated therapy for children with CPP.
Trial registration: ClinicalTrials.gov NCT02452931.
Keywords: central precocious puberty; gonadotropin releasing hormone agonists; leuprolide acetate.
© Endocrine Society 2020. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Bangalore Krishna K, Fuqua JS, Rogol AD, et al. Use of gonadotropin-releasing hormone analogs in children: update by an international consortium. Horm Res Paediatr. 2019; 91(6):357-372. - PubMed
-
- Kletter GB, Klein KO, Wong YY. A pediatrician’s guide to central precocious puberty. Clin Pediatr (Phila). 2015;54(5):414-424. - PubMed
-
- Carel JC, Léger J. Clinical practice. Precocious puberty. N Engl J Med. 2008;358(22):2366-2377. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

