Halogen exposure injury in the developing lung
- PMID: 32738176
- PMCID: PMC9447934
- DOI: 10.1111/nyas.14445
Halogen exposure injury in the developing lung
Abstract
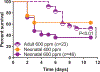
Owing to a high-volume industrial usage of the halogens chlorine (Cl2 ) and bromine (Br2 ), they are stored and transported in abundance, creating a risk for accidental or malicious release to human populations. Despite extensive efforts to understand the mechanisms of toxicity upon halogen exposure and to develop specific treatments that could be used to treat exposed individuals or large populations, until recently, there has been little to no effort to determine whether there are specific features and or the mechanisms of halogen exposure injury in newborns or children. We established a model of neonatal halogen exposure and published our initial findings. In this review, we aim to contrast and compare the findings in neonatal mice exposed to Br2 with the findings published on adult mice exposed to Br2 and the neonatal murine models of bronchopulmonary dysplasia. Despite remarkable similarities across these models in overall alveolar architecture, there are distinct functional and apparent mechanistic differences that are characteristic of each model. Understanding the mechanistic and functional features that are characteristic of the injury process in neonatal mice exposed to halogens will allow us to develop countermeasures that are appropriate for, and effective in, this unique population.
Keywords: alveolar simplification; bromine; bronchopulmonary dysplasia; chlorine; halogen exposure; lung development; newborn.
© 2020 New York Academy of Sciences.
Figures




References
-
- Surate Solaligue DE, Rodriguez-Castillo JA, Ahlbrecht K, et al. 2017. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol 313: L1101–L1153. - PubMed
-
- Hagood JS & Ambalavanan N. 2013. Systems biologyof lung development and regeneration: current knowledge and recommendations for future research. Wiley Interdiscip. Rev. Syst. Biol. Med 5: 125–133. - PubMed
-
- Burri PH 2006. Structural aspects of postnatal lungdevelopment—alveolar formation and growth. Biol. Neonate 89: 313–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

