Mitochondrial Oxidative Damage Underlies Regulatory T Cell Defects in Autoimmunity
- PMID: 32738205
- PMCID: PMC7611060
- DOI: 10.1016/j.cmet.2020.07.001
Mitochondrial Oxidative Damage Underlies Regulatory T Cell Defects in Autoimmunity
Abstract
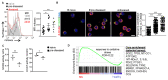
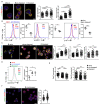
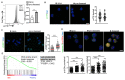
Regulatory T cells (Tregs) are vital for the maintenance of immune homeostasis, while their dysfunction constitutes a cardinal feature of autoimmunity. Under steady-state conditions, mitochondrial metabolism is critical for Treg function; however, the metabolic adaptations of Tregs during autoimmunity are ill-defined. Herein, we report that elevated mitochondrial oxidative stress and a robust DNA damage response (DDR) associated with cell death occur in Tregs in individuals with autoimmunity. In an experimental autoimmune encephalitis (EAE) mouse model of autoimmunity, we found a Treg dysfunction recapitulating the features of autoimmune Tregs with a prominent mtROS signature. Scavenging of mtROS in Tregs of EAE mice reversed the DDR and prevented Treg death, while attenuating the Th1 and Th17 autoimmune responses. These findings highlight an unrecognized role of mitochondrial oxidative stress in defining Treg fate during autoimmunity, which may facilitate the design of novel immunotherapies for diseases with disturbed immune tolerance.
Keywords: DNA damage response; autoimmunity; lysosome; metabolism; mitochondrial oxidative stress; regulatory T cell.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





Comment in
-
Where Mitochondria Meet Autoimmunity: The Treg Cell Link.Cell Metab. 2020 Oct 6;32(4):507-509. doi: 10.1016/j.cmet.2020.08.006. Cell Metab. 2020. PMID: 33027671
References
-
- Alissafi T, Hatzioannou A, Ioannou M, Sparwasser T, Grun JR, Grutzkau A, Verginis P. De novo-induced self-antigen-specific Foxp3+ regulatory T cells impair the accumulation of inflammatory dendritic cells in draining lymph nodes. J Immunol. 2015;194:5812–5824. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

