Obesity-related hypoxia via miR-128 decreases insulin-receptor expression in human and mouse adipose tissue promoting systemic insulin resistance
- PMID: 32739259
- PMCID: PMC7502675
- DOI: 10.1016/j.ebiom.2020.102912
Obesity-related hypoxia via miR-128 decreases insulin-receptor expression in human and mouse adipose tissue promoting systemic insulin resistance
Abstract
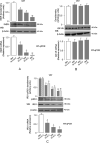
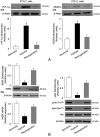
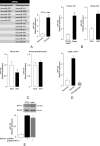
Background: Insulin resistance in visceral adipose tissue (VAT), skeletal muscle and liver is a prominent feature of most patients with obesity. How this association arises remains poorly understood. The objective of this study was to demonstrate that the decrease in insulin receptor (INSR) expression and insulin signaling in VAT from obese individuals is an early molecular manifestation that might play a crucial role in the cascade of events leading to systemic insulin resistance.
Methods: To clarify the role of INSR and insulin signaling in adipose tissue dysfunction in obesity, we first measured INSR expression in VAT samples from normal-weight subjects and patients with different degrees of obesity. We complemented these studies with experiments on high-fat diet (HFD)-induced obese mice, and in human and murine adipocyte cultures, in both normoxic and hypoxic conditions.
Findings: An inverse correlation was observed between increasing body mass index and decreasing INSR expression in VAT of obese humans. Our results indicate that VAT-specific downregulation of INSR is an early event in obesity-related adipose cell dysfunction, which increases systemic insulin resistance in both obese humans and mice. We also provide evidence that obesity-related hypoxia in VAT plays a determinant role in this scenario by decreasing INSR mRNA stability. This decreased stability is through the activation of a miRNA (miR-128) that downregulates INSR expression in adipocytes.
Interpretation: We present a novel pathogenic mechanism of reduced INSR expression and insulin signaling in adipocytes. Our data provide a new explanation linking obesity with systemic insulin resistance.
Funding: This work was partly supported by a grant from Nutramed (PON 03PE000_78_1) and by the European Commission (FESR FSE 2014-2020 and Regione Calabria).
Keywords: Adipose-tissue dysfunction; Hypoxia; Insulin receptor; Insulin resistance; Obesity; mRNA decay; miRNA.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflicts of interest.
Figures





References
-
- Flegal K.M., Carroll M.D., Kit B.K., Ogden C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. J Am Med Assoc. 2012;307:491–497. - PubMed
-
- Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. - PubMed
-
- Reaven G.M. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. - PubMed
-
- Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

