Sterol-O-acyltransferase-1 has a role in kidney disease associated with diabetes and Alport syndrome
- PMID: 32739420
- PMCID: PMC7606642
- DOI: 10.1016/j.kint.2020.06.040
Sterol-O-acyltransferase-1 has a role in kidney disease associated with diabetes and Alport syndrome
Abstract
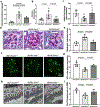
Defective cholesterol metabolism primarily linked to reduced ATP-binding cassette transporter A1 (ABCA1) expression is closely associated with the pathogenesis and progression of kidney diseases, including diabetic kidney disease and Alport Syndrome. However, whether the accumulation of free or esterified cholesterol contributes to progression in kidney disease remains unclear. Here, we demonstrate that inhibition of sterol-O-acyltransferase-1 (SOAT1), the enzyme at the endoplasmic reticulum that converts free cholesterol to cholesterol esters, which are then stored in lipid droplets, effectively reduced cholesterol ester and lipid droplet formation in human podocytes. Furthermore, we found that inhibition of SOAT1 in podocytes reduced lipotoxicity-mediated podocyte injury in diabetic kidney disease and Alport Syndrome in association with increased ABCA1 expression and ABCA1-mediated cholesterol efflux. In vivo, Soat1 deficient mice did not develop albuminuria or mesangial expansion at 10-12 months of age. However, Soat1 deficiency/inhibition in experimental models of diabetic kidney disease and Alport Syndrome reduced cholesterol ester content in kidney cortices and protected from disease progression. Thus, targeting SOAT1-mediated cholesterol metabolism may represent a new therapeutic strategy to treat kidney disease in patients with diabetic kidney disease and Alport Syndrome, like that suggested for Alzheimer's disease and cancer treatments.
Keywords: Alport syndrome; SOAT1 inhibitor; cholesterol; diabetic kidney disease; podocyte injury; renal function.
Copyright © 2020 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures
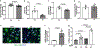

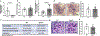
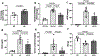

Comment in
-
The ins-and-outs of podocyte lipid metabolism.Kidney Int. 2020 Nov;98(5):1087-1090. doi: 10.1016/j.kint.2020.07.008. Kidney Int. 2020. PMID: 33126971
References
-
- Proctor G, Jiang T, Iwahashi M, et al. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 2006; 55: 2502–2509. - PubMed
-
- Nosadini R, Tonolo G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr Metab Cardiovasc Dis 2011; 21: 79–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

