The pathophysiology of SARS-CoV-2: A suggested model and therapeutic approach
- PMID: 32739471
- PMCID: PMC7392886
- DOI: 10.1016/j.lfs.2020.118166
The pathophysiology of SARS-CoV-2: A suggested model and therapeutic approach
Abstract
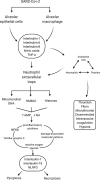
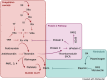
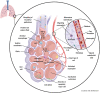
In this paper, a model is proposed of the pathophysiological processes of COVID-19 starting from the infection of human type II alveolar epithelial cells (pneumocytes) by SARS-CoV-2 and culminating in the development of ARDS. The innate immune response to infection of type II alveolar epithelial cells leads both to their death by apoptosis and pyroptosis and to alveolar macrophage activation. Activated macrophages secrete proinflammatory cytokines and chemokines and tend to polarise into the inflammatory M1 phenotype. These changes are associated with activation of vascular endothelial cells and thence the recruitment of highly toxic neutrophils and inflammatory activated platelets into the alveolar space. Activated vascular endothelial cells become a source of proinflammatory cytokines and reactive oxygen species (ROS) and contribute to the development of coagulopathy, systemic sepsis, a cytokine storm and ARDS. Pulmonary activated platelets are also an important source of proinflammatory cytokines and ROS, as well as exacerbating pulmonary neutrophil-mediated inflammatory responses and contributing to systemic sepsis by binding to neutrophils to form platelet-neutrophil complexes (PNCs). PNC formation increases neutrophil recruitment, activation priming and extraversion of these immune cells into inflamed pulmonary tissue, thereby contributing to ARDS. Sequestered PNCs cause the development of a procoagulant and proinflammatory environment. The contribution to ARDS of increased extracellular histone levels, circulating mitochondrial DNA, the chromatin protein HMGB1, decreased neutrophil apoptosis, impaired macrophage efferocytosis, the cytokine storm, the toll-like receptor radical cycle, pyroptosis, necroinflammation, lymphopenia and a high Th17 to regulatory T lymphocyte ratio are detailed.
Keywords: COVID-19; Respiratory infection; SARS-CoV-2; Treatment.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest MB is supported by a NHMRC Senior Principal Research Fellowship (1059660 and 1156072). MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Astra Zeneca, Lundbeck, Merck, Pfizer, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Lundbeck Merck, Pfizer and Servier – all unrelated to this work. LO is supported by a NHMRC Early Career Fellowship (1158487). WM is currently funded by an Alfred Deakin Postdoctoral Research Fellowship and a Multiple Sclerosis Research Australia early-career fellowship. WM has previously received funding from the Cancer Council Queensland and university grants/fellowships from La Trobe University, Deakin University, University of Queensland, and Bond University. WM has received industry funding and has attended events funded by Cobram Estate Pty. Ltd. WM has received travel funding from Nutrition Society of Australia. WM has received consultancy funding from Nutrition Research Australia. WM has received speaker honoraria from The Cancer Council Queensland and the Princess Alexandra Research Foundation. The Food & Mood Centre has received Grant/Research support from Fernwood Foundation, Wilson Foundation, the A2 Milk Company, and Be Fit Foods. AO is supported by a Future Leader Fellowship (#101160) from the Heart Foundation Australia and Wilson Foundation. She has received research funding from National Health & Medical Research Council, Australian Research Council, University of Melbourne, Deakin University, Sanofi, Meat and Livestock Australia and Woolworths Limited and Honoraria from Novartis.
Figures

References
-
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

