Development and validation of a novel scoring system developed from a nomogram to identify malignant pleural effusion
- PMID: 32739872
- PMCID: PMC7393523
- DOI: 10.1016/j.ebiom.2020.102924
Development and validation of a novel scoring system developed from a nomogram to identify malignant pleural effusion
Abstract
Background: This study aimed to establish and validate a novel scoring system based on a nomogram for the differential diagnosis of malignant pleural effusion (MPE) and benign pleural effusion (BPE).
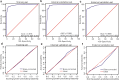
Methods: Patients with PE and confirmed aetiology who underwent diagnostic thoracentesis were included in this study. One retrospective set (N = 1261) was used to develop and internally validate the predictive model. The clinical, radiological and laboratory features were collected and subjected to logistic regression analyses. The primary predictive model was displayed as a nomogram and then modified into a novel scoring system, which was externally validated in an independent set (N = 172).
Findings: The novel scoring system was composed of fever (3 points), erythrocyte sedimentation rate (4 points), effusion adenosine deaminase (7 points), serum carcinoembryonic antigen (CEA) (4 points), effusion CEA (10 points) and effusion/serum CEA (8 points). With a cutoff value of 15 points, the area under the curve, specificity and sensitivity for identifying MPE were 0.913, 89.10%, and 82.63%, respectively, in the training set, 0.922, 93.48%, 81.51%, respectively, in the internal validation set and 0.912, 87.61%, 81.36%, respectively, in the external validation set. Moreover, this scoring system was exclusively applied to distinguish lung cancer with PE from tuberculous pleurisy and showed a favourable diagnostic performance in the training and validation sets.
Interpretation: This novel scoring system was developed from a retrospective study and externally validated in an independent set based on six easily accessible clinical variables, and it exhibited good diagnostic performance for identifying MPE.
Funding: NFSC grants (no. 81572942, no. 81800094).
Keywords: Clinical features; Diagnostic value; Malignant pleural effusion; Nomogram; Scoring system.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declarations of Competing Interests All authors have no competing interests to report.
Figures




References
-
- Feller-Kopman D., Light R. Pleural Disease. N Engl J Med. 2018;378:740–751. - PubMed
-
- Ferreiro L., Toubes M.E., San José M.E., Suárez-Antelo J., Golpe A., Valdés L. Advances in pleural effusion diagnostics. Expert Rev Respir Med. 2020;14:51–66. - PubMed
-
- Taghizadeh N., Fortin M., Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 national inpatient sample. Chest. 2017;151 845-4. - PubMed
-
- Wang G., Wang S., Yang X., Sun Q., Jiang G., Huang M. Accuracy of Xpert MTB/RIF ultra for the diagnosis of pleural TB in a multicenter cohort study. Chest. 2019 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

