The rebirth of the contact pathway: a new therapeutic target
- PMID: 32740037
- PMCID: PMC7596882
- DOI: 10.1097/MOH.0000000000000603
The rebirth of the contact pathway: a new therapeutic target
Abstract
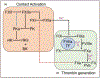
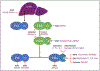
Purpose of review: Anticoagulation with vitamin-K antagonists or direct oral anticoagulants is associated with a significant risk of bleeding. There is a major effort underway to develop antithrombotic drugs that have a smaller impact on hemostasis. The plasma contact proteins factor XI (FXI) and factor XII (FXII) have drawn considerable interest because they contribute to thrombosis but have limited roles in hemostasis. Here, we discuss results of preclinical and clinical trials supporting the hypothesis that the contact system contributes to thromboembolic disease.
Recent findings: Numerous compounds targeting FXI or FXII have shown antithrombotic properties in preclinical studies. In phase 2 studies, drugs-targeting FXI or its protease form FXIa compared favorably with standard care for venous thrombosis prophylaxis in patients undergoing knee replacement. While less work has been done with FXII inhibitors, they may be particularly useful for limiting thrombosis in situations where blood comes into contact with artificial surfaces of medical devices.
Summary: Inhibitors of contact activation, and particularly of FXI, are showing promise for prevention of thromboembolic disease. Larger studies are required to establish their efficacy, and to establish that they are safer than current therapy from a bleeding standpoint.
Conflict of interest statement
Conflicts of interest
P.S. has no conflict to report. D.S. is a consultant for several pharmaceutical companies that are developing compounds that target factor XI and factor XII for therapeutic purposes.
Figures
References
-
- Simon EM, Streitz MJ, Sessions DJ, Kaide CG. Anticoagulation reversal. Emerg Med Clin North Am 2018; 36:585–601. - PubMed
-
- van Es N, Coppens M, Schulman S, et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014; 124:1968–1975. - PubMed
-
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352. - PubMed
-
- Khorana AA, Noble S, Lee AYY, et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost 2018; 16:1891–1894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

