Tuberculosis and viral hepatitis in patients treated with certolizumab pegol in Asia-Pacific countries and worldwide: real-world and clinical trial data
- PMID: 32740672
- PMCID: PMC7895783
- DOI: 10.1007/s10067-020-05248-4
Tuberculosis and viral hepatitis in patients treated with certolizumab pegol in Asia-Pacific countries and worldwide: real-world and clinical trial data
Erratum in
-
Correction to: Tuberculosis and viral hepatitis in patients treated with certolizumab pegol in Asia-Pacific countries and worldwide: real-world and clinical trial data.Clin Rheumatol. 2020 Oct;39(10):3161. doi: 10.1007/s10067-020-05356-1. Clin Rheumatol. 2020. PMID: 32876785 Free PMC article.
Abstract
Introduction/objectives: To evaluate the incidence rate (IR) of tuberculosis (TB) and viral hepatitis B and C (HBV/HCV) during certolizumab pegol (CZP) treatment, worldwide and in Asia-Pacific countries, across clinical trials and post-marketing reports (non-interventional studies and real-world practice).
Method: CZP safety data were pooled across 49 clinical trials from 1998 to June 2017. Post-marketing reports were from initial commercialization until March 2015 (TB)/February 2017 (HBV/HCV). All suspected TB and HBV/HCV cases underwent centralized retrospective review by external experts. Incidence rates (IRs) were calculated per 100 patient-years (PY) of CZP exposure.
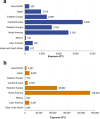
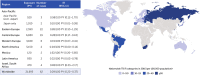
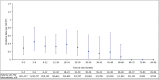
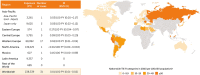
Results: Among 11,317 clinical trial patients (21,695 PY), 62 TB cases were confirmed (IR 0.29/100 PY) including 2 in Japan (0.10/100 PY) and 3 in other Asia-Pacific countries (0.58/100 PY). From > 238,000 PY estimated post-marketing CZP exposure, there were 31 confirmed TB cases (0.01/100 PY): 5 in Japan (0.05/100 PY), 1 in other Asia-Pacific countries (0.03/100 PY). Reported regional TB IRs were highest in eastern Europe (0.17/100 PY), central Europe (0.09/100 PY), and Mexico (0.16/100 PY). Across clinical trials, there was 1 confirmed HBV reactivation and no HCV cases. From > 420,000 PY estimated post-marketing CZP exposure, 5 HBV/HCV cases were confirmed (0.001/100 PY): 2 HCV reactivations; 1 new HCV; plus 2 HBV reactivations in Japan (0.008/100 PY).
Conclusions: CZP TB risk is aligned with nationwide TB rates, being slightly higher in Asia-Pacific countries excluding Japan. Overall, TB and HBV/HCV risk with CZP treatment is currently relatively low, as risk can be minimized with patient/physician education, screening, and vigilant treatment, according to international guidelines.
Key points: • TB rates were highest in eastern/central Europe, Mexico, and Asia-Pacific regions. • With the implementation of stricter TB screening and risk evaluations in 2007, especially in high TB incidence countries, there was a notable reduction TB occurrence. • Safety profile of biologics in real-world settings complements controlled studies. • TB and hepatitis (HBV/HCV) risk with certolizumab pegol (CZP) treatment is low.
Keywords: Pharmacovigilance; Psoriatic arthritis; Rheumatic diseases; Rheumatoid arthritis; Spondyloarthritis.
Conflict of interest statement
CSL: Consulting fees from UCB Pharma; YHC: Consulting fees from UCB Pharma; KL: Consulting fees from UCB Pharma; MdL: Former employee of UCB Pharma; CA: Employee of UCB Pharma; KW: Consulting fees from AbbVie, Bristol-Myers Squibb, Eli-Lilly, Roche, Pfizer, and UCB Pharma.
Figures

References
-
- Mariette X, Vencovsky J, Lortholary O, Gomez-Reino J, de Longueville M, Ralston P, Weinblatt M, van Vollenhoven R. The incidence of tuberculosis in patients treated with certolizumab pegol across indications: impact of baseline skin test results, more stringent screening criteria and geographic region. RMD Open. 2015;1:e000044. doi: 10.1136/rmdopen-2014-000044. - DOI - PMC - PubMed
-
- Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, Kampmann B, Hellmich B, Groves R, Schreiber S, Wallis RS, Sotgiu G, Scholvinck EH, Goletti D, Zellweger JP, Diel R, Carmona L, Bartalesi F, Ravn P, Bossink A, Duarte R, Erkens C, Clark J, Migliori GB, Lange C. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010;36:1185–1206. doi: 10.1183/09031936.00028510. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

