Ten-Eleven Translocation 1 Promotes Malignant Progression of Cholangiocarcinoma With Wild-Type Isocitrate Dehydrogenase 1
- PMID: 32740973
- PMCID: PMC7855500
- DOI: 10.1002/hep.31486
Ten-Eleven Translocation 1 Promotes Malignant Progression of Cholangiocarcinoma With Wild-Type Isocitrate Dehydrogenase 1
Abstract
Background and aims: Cholangiocarcinoma (CCA) is a highly lethal disease without effective therapeutic approaches. The whole-genome sequencing data indicate that about 20% of patients with CCA have isocitrate dehydrogenase 1 (IDH1) mutations, which have been suggested to target 2-oxoglutarate (OG)-dependent dioxygenases in promoting CCA carcinogenesis. However, the clinical study indicates that patients with CCA and mutant IDH1 have better prognosis than those with wild-type IDH1, further complicating the roles of 2-OG-dependent enzymes.
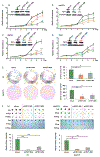
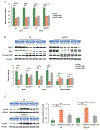
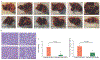
Approach and results: This study aimed to clarify if ten-eleven translocation 1 (TET1), which is one of the 2-OG-dependent enzymes functioning in regulating 5-hydroxymethylcytosine (5hmC) formation, is involved in CCA progression. By analyzing The Cancer Genome Atlas (TCGA) data set, TET1 mRNA was found to be substantially up-regulated in patients with CCA when compared with noncancerous bile ducts. Additionally, TET1 protein expression was significantly elevated in human CCA tumors. CCA cells were challenged with α-ketoglutarate (α-KG) and dimethyl-α-KG (DM-α-KG), which are cosubstrates for TET1 dioxygenase. The treatments with α-KG and DM-α-KG promoted 5hmC formation and malignancy of CCA cells. Molecular and pharmacological approaches were used to inhibit TET1 activity, and these treatments substantially suppressed 5hmC and CCA carcinogenesis. Mechanistically, it was found that knockdown of TET1 may suppress CCA progression by targeting cell growth and apoptosis through epigenetic regulation. Consistently, targeting TET1 significantly inhibited CCA malignant progression in a liver orthotopic xenograft model by targeting cell growth and apoptosis.
Conclusions: Our data suggest that expression of TET1 is highly associated with CCA carcinogenesis. It will be important to evaluate TET1 expression in CCA tumors before application of the IDH1 mutation inhibitor because the inhibitor suppresses 2-hydroxyglutarate expression, which may result in activation of TET, potentially leading to CCA malignancy.
© 2021 by the American Association for the Study of Liver Diseases.
Figures




References
-
- Chan-On W, Nairismagi ML, Ong CK, Lim WK, Dima S, Pairojkul C, Lim KH, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet 2013;45:1474–1478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

