Evaluation of impaired growth plate development of long bones in skeletally immature mice by antirheumatic agents
- PMID: 32740982
- PMCID: PMC7984053
- DOI: 10.1002/jor.24819
Evaluation of impaired growth plate development of long bones in skeletally immature mice by antirheumatic agents
Abstract
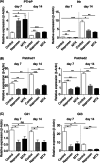
Restriction of physical growth and development is a known problem in patients with juvenile idiopathic arthritis (JIA). However, the effect of medical treatment for JIA on skeletal growth in affected children has not been properly investigated. We, therefore, hypothesize that naproxen and methotrexate (MTX) affect endochondral ossification and will lead to reduced skeletal development. Treatment of ATDC5 cells, an in vitro model for endochondral ossification, with naproxen or MTX resulted in increased chondrogenic but decreased hypertrophic differentiation. In vivo, healthy growing C57BL/6 mice were treated with naproxen, MTX, or placebo for 10 weeks. At 15 weeks postnatal, both the length of the tibia and the length of the femur were significantly reduced in the naproxen- and MTX-treated mice compared to their controls. Growth plate analysis revealed a significantly thicker proliferative zone, while the hypertrophic zone was significantly thinner in both experimental groups compared to their controls, comparable to the in vitro results. Micro-computed tomography analysis of the subchondral bone region directly below the growth disc showed significantly altered bone microarchitecture in naproxen and MTX groups. In addition, the involvement of the PTHrP-Ihh loop in naproxen- and MTX-treated cells was shown. Overall, these results demonstrate that naproxen and MTX have a profound effect on endochondral ossification during growth plate development, abnormal subchondral bone morphology, and reduced bone length. A better understanding of how medication influences the development of the growth plate will improve understanding of endochondral ossification and reveal possibilities to improve the treatment of pediatric patients.
Keywords: JIA; MTX; endochondral ossification; growth plate; naproxen.
© 2020 The Authors. Journal of Orthopaedic Research ® published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures





References
-
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332‐336. - PubMed
-
- Chung R, Xian CJ. Recent research on the growth plate: mechanisms for growth plate injury repair and potential cell‐based therapies for regeneration. J Mol Endocrinol. 2014;53:T45‐T61. - PubMed
-
- Politoolito C, Strano CG, Olivieri AN, et al. Growth retardation in non‐steroid treated juvenile rheumatoid arthritis. Scand J Rheumatol. 1997;26:99‐103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

