The Use of Aerosolized Ribavirin in Respiratory Syncytial Virus Lower Respiratory Tract Infections in Adult Immunocompromised Patients: A Systematic Review
- PMID: 32742010
- PMCID: PMC7370350
- DOI: 10.1177/0018578719836646
The Use of Aerosolized Ribavirin in Respiratory Syncytial Virus Lower Respiratory Tract Infections in Adult Immunocompromised Patients: A Systematic Review
Abstract
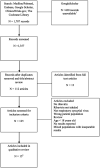
Introduction: Respiratory syncytial virus (RSV)-associated lower respiratory tract infection (LRTI) is a concern in immunocompromised patients. Aerosolized ribavirin (RBV AER) is used for treatment of RSV LRTI; however, adverse events and rising drug costs remain a challenge for patient management. The purpose of this systematic review is to summarize the efficacy and adverse event profile of RBV AER for the treatment of hospitalized RSV LRTI in immunocompromised adult patients. Methods: A Medline/PubMed, Embase, Google Scholar, Clinicaltrials.gov, and Cochrane Library database search was conducted from 1966 to January 2019 for the use of RBV AER. Search strategy: [(ribavirin OR ICN1229) AND ("administration, oral" OR "oral" OR "administration, inhalation" OR "inhalation)] AND ("respiratory tract infection" OR "pneumonia"). Studies were reviewed if adult patients were hospitalized, immunocompromised, had RSV LRTI, received RBV AER, and included the outcome of mortality and/or adverse reactions. Methodological quality was assessed using the Cochrane Collaboration GRADE approach. Results: A total of 1787 records were identified and 15 articles met inclusion criteria: hematopoietic stem cell transplant (HSCT)/bone marrow transplant (n = 8), other malignancy/neutropenic (n = 2), solid organ transplant (n = 5). All of the trials are observational with a low quality rating; therefore, a meta-analysis was not performed. The 30-day mortality in studies that contain >10 patients with HSCT, malignancy, and transplant range from 0 to 15.4%, 6.3%, and 0 to 27%, respectively. Improved mortality was cited in 4 studies when RBV AER started before mechanical ventilation or within 2 weeks of symptom onset. Only 3 studies had comparative mortality data with RBV AER and RBV PO. Adverse reactions were reported in 5 studies and included psychiatric manifestations (anxiety, depression, feeling of isolation; n = 14), wheezing/bronchospasm (n = 6), snowflakes/hail blowing in face (n = 6), and precipitation in ventilator tubing (n = 5). Conclusion: There is a lack of high quality, comparative trials on the use of RBV AER for the treatment of RSV LRTI in adult hospitalized immunocompromised patients. There may be a mortality benefit when RBV AER is initiated early after diagnosis or prior to mechanical ventilation, but requires further study. Patient isolation and psychological effects must be weighed against the benefit of therapy.
Keywords: immunocompromised; lower respiratory tract infection; respiratory syncytial virus; ribavirin; systematic review.
© The Author(s) 2019.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products. Date unknown. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed January 23, 2019.
LinkOut - more resources
Full Text Sources
Miscellaneous

