This is a preprint.
The Loss of Bcl-6 Expressing T Follicular Helper Cells and the Absence of Germinal Centers in COVID-19
- PMID: 32742244
- PMCID: PMC7385482
- DOI: 10.2139/ssrn.3652322
The Loss of Bcl-6 Expressing T Follicular Helper Cells and the Absence of Germinal Centers in COVID-19
Update in
-
Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19.Cell. 2020 Oct 1;183(1):143-157.e13. doi: 10.1016/j.cell.2020.08.025. Epub 2020 Aug 19. Cell. 2020. PMID: 32877699 Free PMC article.
Abstract
Humoral responses in COVID-19 disease are often of limited durability, as seen with other human coronavirus epidemics. To address the underlying etiology, we examined postmortem thoracic lymph nodes and spleens in acute SARS-CoV-2 infection and observed the absence of germinal centers, a striking reduction in Bcl-6+ germinal center B cells but preservation of AID+ B cells. Absence of germinal centers correlated with an early specific block in Bcl-6+TFH cell differentiation together with an increase in T-bet+TH1 cells and aberrant extra-follicular TNF-a accumulation. Parallel peripheral blood studies revealed loss of transitional and follicular B cells in severe disease and accumulation of SARS-CoV-2-specific "disease-related" B cell populations. These data identify defective Bcl-6+TFH cell generation and dysregulated humoral immune induction early in COVID-19 disease, providing a mechanistic explanation for the limited durability of antibody responses in coronavirus infections and suggest that achieving herd immunity through natural infection may be difficult. Funding: This work was supported by NIH U19 AI110495 to SP, NIH R01 AI146779 to AGS, NIH R01AI137057 and DP2DA042422 to DL, BMH was supported by NIGMS T32 GM007753, TMC was supported by T32 AI007245. Funding for these studies from the Massachusetts Consortium of Pathogen Readiness, the Mark and Lisa Schwartz Foundation and Enid Schwartz is also acknowledged. Conflict of Interest: None. Ethical Approval: This study was performed with the approval of the Institutional Review Boards at the Massachusetts General Hospital and the Brigham and Women's Hospital.
Figures
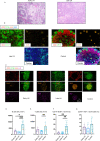
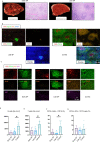
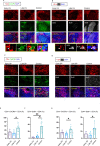
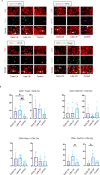


Comment in
-
Getting to the (germinal) centre of SARS-CoV-2.Nat Rev Immunol. 2020 Oct;20(10):590. doi: 10.1038/s41577-020-00424-8. Nat Rev Immunol. 2020. PMID: 32782355 Free PMC article. No abstract available.
References
-
- Buja L.M., Wolf D.A., Zhao B., Akkanti B., McDonald M., Lelenwa L., Reilly N., Ottaviani G., Elghetany M.T., Trujillo D.O., et al. (2020). The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol 48, 107233. - PMC - PubMed
-
- Cao W.C., Liu W., Zhang P.H., Zhang F., and Richardus J.H. (2007). Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med 357, 1162–1163. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
