Nascent teichoic acids insertion into the cell wall directs the localization and activity of the major pneumococcal autolysin LytA
- PMID: 32743129
- PMCID: PMC7389264
- DOI: 10.1016/j.tcsw.2018.05.001
Nascent teichoic acids insertion into the cell wall directs the localization and activity of the major pneumococcal autolysin LytA
Abstract
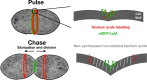
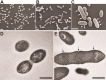
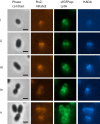
The bacterial cell wall is in part composed of the peptidoglycan (PG) layer that maintains the cell shape and sustains the basic cellular processes of growth and division. The cell wall of Gram-positive bacteria also carries teichoic acids (TAs). In this work, we investigated how TAs contribute to the structuration of the PG network through the modulation of PG hydrolytic enzymes in the context of the Gram-positive Streptococcus pneumoniae bacterium. Pneumococcal TAs are decorated by phosphorylcholine residues which serve as anchors for the Choline-Binding Proteins, some of them acting as PG hydrolases, like the major autolysin LytA. Their binding is non covalent and reversible, a property that allows easy manipulation of the system. In this work, we show that the release of LytA occurs independently from its amidase activity. Furthermore, LytA fused to GFP was expressed in pneumococcal cells and showed different localization patterns according to the growth phase. Importantly, we demonstrate that TAs modulate the enzymatic activity of LytA since a low level of TAs present at the cell surface triggers LytA sensitivity in growing pneumococcal cells. We previously developed a method to label nascent TAs in live cells revealing that the insertion of TAs into the cell wall occurs at the mid-cell. In conclusion, we demonstrate that nascent TAs inserted in the cell wall at the division site are the specific receptors of LytA, tuning in this way the positioning of LytA at the appropriate place at the cell surface.
Keywords: Choline-Binding Proteins; Major autolysin LytA localization; Peptidoglycan hydrolases; Streptococcus pneumoniae cell wall; Teichoic acids localization.
© 2018 Published by Elsevier B.V.
Figures







References
-
- Bonnet J., Durmort C., Jacq M., Mortier-Barrière I., Campo N., VanNieuwenhze M.S., Brun Y.V., Arthaud C., Gallet B., Moriscot C., Morlot C., Vernet T., Di Guilmi A.M. Peptidoglycan O-acetylation is functionally related to cell wall biosynthesis and cell division in Streptococcus pneumoniae. Mol. Microbiol. 2017;106:832–846. - PMC - PubMed
LinkOut - more resources
Full Text Sources

