PRMT5 function and targeting in cancer
- PMID: 32743345
- PMCID: PMC7380451
- DOI: 10.15698/cst2020.08.228
PRMT5 function and targeting in cancer
Abstract
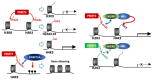
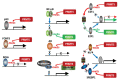
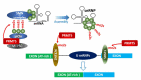
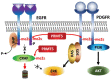
Protein methyl transferases play critical roles in numerous regulatory pathways that underlie cancer development, progression and therapy-response. Here we discuss the function of PRMT5, a member of the nine-member PRMT family, in controlling oncogenic processes including tumor intrinsic, as well as extrinsic microenvironmental signaling pathways. We discuss PRMT5 effect on histone methylation and methylation of regulatory proteins including those involved in RNA splicing, cell cycle, cell death and metabolic signaling. In all, we highlight the importance of PRMT5 regulation and function in cancer, which provide the foundation for therapeutic modalities targeting PRMT5.
Keywords: MEP50; PRMT1; PRMT5; histone; methylation; methyltransferase; splicing; transcription.
Copyright: © 2020 Kim and Ronai.
Conflict of interest statement
Conflict of interest: ZR is a co-founder and serves as scientific advisor to Pangea Therapeutics. HK declares no competing interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
