Immunometabolism in the pathogenesis of systemic lupus erythematosus
- PMID: 32743527
- PMCID: PMC7388408
- DOI: 10.1016/j.jtauto.2020.100046
Immunometabolism in the pathogenesis of systemic lupus erythematosus
Abstract
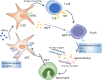
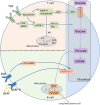
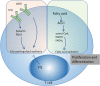
Systemic lupus erythematosus (SLE) is a typical autoimmune disease characterized by chronic inflammation and pathogenic auto-antibodies. Apart from B cells, dysregulation of other immune cells also plays an essential role in the pathogenesis and development of the disease including CD4+T cells, dendritic cells, macrophages and neutrophils. Since metabolic programs control immune cell fate and function, they are critical checkpoints in an effective immune response and are involved in the etiology of autoimmune disease. In addition, mitochondria and oxidative stress are both involved in cellular metabolism and is also essential in immune response. In this review, apart from the disturbed immune system, we will discuss mitochondrial dysfunction, oxidative stress, abnormal metabolism (including glucose, lipid and amino acid metabolism) of immune cells as well as epigenetic control of metabolism reprogramming to elucidate the underlying pathogenic mechanisms of systemic lupus erythematosus.
Keywords: Immune response; Metabolic programs; Pathogenesis; Systemic lupus erythematosus (SLE).
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

