Methionine oxidation within the prion protein
- PMID: 32744136
- PMCID: PMC7518762
- DOI: 10.1080/19336896.2020.1796898
Methionine oxidation within the prion protein
Abstract
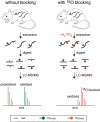
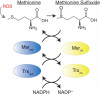
Prion diseases are characterized by the self-templated misfolding of the cellular prion protein (PrPC) into infectious aggregates (PrPSc). The detailed molecular basis of the misfolding and aggregation of PrPC remains incompletely understood. It is believed that the transient misfolding of PrPC into partially structured intermediates precedes the formation of insoluble protein aggregates and is a critical component of the prion misfolding pathway. A number of environmental factors have been shown to induce the destabilization of PrPC and promote its initial misfolding. Recently, oxidative stress and reactive oxygen species (ROS) have emerged as one possible mechanism by which the destabilization of PrPC can be induced under physiological conditions. Methionine residues are uniquely vulnerable to oxidation by ROS and the formation of methionine sulfoxides leads to the misfolding and subsequent aggregation of PrPC. Here, we provide a review of the evidence for the oxidation of methionine residues in PrPC and its potential role in the formation of pathogenic prion aggregates.
Keywords: Prions; methionine; oxidation; protein aggregation; protein misfolding.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

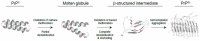
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
