Novel chimerized IgA CD20 antibodies: Improving neutrophil activation against CD20-positive malignancies
- PMID: 32744145
- PMCID: PMC7531568
- DOI: 10.1080/19420862.2020.1795505
Novel chimerized IgA CD20 antibodies: Improving neutrophil activation against CD20-positive malignancies
Abstract
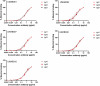
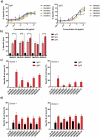
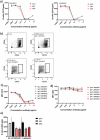
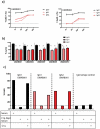
Current combination therapies elicit high response rates in B cell malignancies, often using CD20 antibodies as the backbone of therapy. However, many patients eventually relapse or develop progressive disease. Therefore, novel CD20 antibodies combining multiple effector mechanisms were generated. To study whether neutrophil-mediated destruction of B cell malignancies can be added to the arsenal of effector mechanisms, we chimerized a panel of five previously described murine CD20 antibodies to the human IgG1, IgA1 and IgA2 isotype. Of this panel, we assessed in vitro antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and direct cell death induction capacity and studied the efficacy in two different in vivo mouse models. IgA antibodies outperformed IgG1 antibodies in neutrophil-mediated killing in vitro, both against CD20-expressing cell lines and primary patient material. In these assays, we observed loss of CD19 with both IgA and IgG antibodies. Therefore, we established a novel method to improve the assessment of B-cell depletion by CD20 antibodies by including CD24 as a stable cell marker. Subsequently, we demonstrated that only IgA antibodies were able to reduce B cell numbers in this context. Additionally, IgA antibodies showed efficacy in both an intraperitoneal tumor model with EL4 cells expressing huCD20 and in an adoptive transfer model with huCD20-expressing B cells. Taken together, we show that IgA, like IgG, can induce ADCC and CDC, but additionally triggers neutrophils to kill (malignant) B cells. We conclude that antibodies of the IgA isotype offer an attractive repertoire of effector mechanisms for the treatment of CD20-expressing malignancies.
Keywords: ADCC; CD20; CDC; IgA; antibodies; apoptosis.
Figures


References
-
- Mossner E, Brünker P, Moser S, Püntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell–mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–10. doi:10.1182/blood-2009-06-225979. - DOI - PMC - PubMed
-
- Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, Pocock C, Ardeshna KM, Radford JA, McMillan A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817–26. doi:10.1016/S0140-6736(13)60313-X. - DOI - PubMed
-
- Vitolo U, Trněný M, Belada D, Burke JM, Carella AM, Chua N, Abrisqueta P, Demeter J, Flinn I, Hong X, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-Cell Lymphoma. J Clin Oncol. 2017;35(31):3529–37. doi:10.1200/JCO.2017.73.3402. - DOI - PubMed
-
- Wilson WH, Jung S-H, Porcu P, Hurd D, Johnson J, Martin SE, Czuczman M, Lai R, Said J, Chadburn A, et al. A cancer and leukemia group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. 2012;97(5):758–65. doi:10.3324/haematol.2011.056531. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
