Development and validation of an LC-MS/MS method for simultaneous quantification of co-administered trastuzumab and pertuzumab
- PMID: 32744170
- PMCID: PMC7531571
- DOI: 10.1080/19420862.2020.1795492
Development and validation of an LC-MS/MS method for simultaneous quantification of co-administered trastuzumab and pertuzumab
Abstract
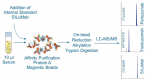
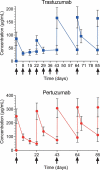
Given the increasing use of combination therapy with multiple monoclonal antibodies (mAbs), there is a clinical need for multiplexing assays. For the frequently co-administered anti-human epidermal growth factor receptor 2 (HER2) mAbs trastuzumab and pertuzumab, we developed a high-throughput and robust hybrid ligand-binding liquid chromatography-mass spectrometry (LC-MS)/MS quantitative assay. Nanomolar concentrations of trastuzumab and pertuzumab were determined in 10 µL serum samples after extraction by affinity purification through protein A beads, followed by on-bead reduction, alkylation, and trypsin digestion. After electrospray ionization, quantification was obtained by multiple reaction monitoring LC-MS/MS using SILuMab as an internal standard. The method was validated according to the current guidelines from the US Food and Drug Administration and the European Medicines Agency. Assay linearity was established in the ranges 0.250-250 μg/mL for trastuzumab and 0.500-500 μg/mL for pertuzumab. The method was accurate and selective for the simultaneous determination of trastuzumab and pertuzumab in clinical samples, thereby overcoming the limitation of ligand binding assays that cannot quantify mAbs targeting the same receptor. Furthermore, this method requires a small blood volume, which reduces blood collection time and stress for patients. The assay robustness was verified in a clinical trial where trastuzumab and pertuzumab concentrations were determined in 670 serum samples. As we used commercially available reagents and standards, the described generic bioanalytical strategy can easily be adapted to multiplex quantifications of other mAb combinations in non-clinical and clinical samples.
Keywords: Trastuzumab; lc-MS; mAbs; monoclonal antibodies; multiplex; pertuzumab; pharmacokinetics; validation.
Figures
Similar articles
-
Simultaneous quantification of co-administered trastuzumab and pertuzumab in serum based on nano-surface and molecular-orientation limited (nSMOL) proteolysis.RSC Adv. 2024 Jun 18;14(27):19550-19559. doi: 10.1039/d4ra03060e. eCollection 2024 Jun 12. RSC Adv. 2024. PMID: 38895524 Free PMC article.
-
Method development and validation of LC-MS/MS-based assay for the simultaneous quantitation of trastuzumab and pertuzumab in cynomolgus monkey serum and its application in pharmacokinetic study.Biomed Chromatogr. 2020 Dec;34(12):e4903. doi: 10.1002/bmc.4903. Epub 2020 Sep 28. Biomed Chromatogr. 2020. PMID: 32428305
-
Analytical and pharmacological consequences of the in vivo deamidation of trastuzumab and pertuzumab.Anal Bioanal Chem. 2022 Feb;414(4):1513-1524. doi: 10.1007/s00216-021-03756-z. Epub 2022 Jan 10. Anal Bioanal Chem. 2022. PMID: 35001193
-
Pertuzumab for the treatment of human epidermal growth factor receptor type 2-positive metastatic breast cancer.Am J Health Syst Pharm. 2013 Sep 15;70(18):1579-87. doi: 10.2146/ajhp120735. Am J Health Syst Pharm. 2013. PMID: 23988598 Review.
-
Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with HER2-positive breast cancer: A multidisciplinary approach.J Oncol Pharm Pract. 2021 Jul;27(5):1214-1221. doi: 10.1177/1078155221999712. Epub 2021 Mar 9. J Oncol Pharm Pract. 2021. PMID: 33719721 Review.
Cited by
-
Analysis of 2'-hydroxyflavanone (2HF) in mouse whole blood by HPLC-MS/MS for the determination of pharmacokinetic parameters.Front Chem. 2023 Mar 9;11:1016193. doi: 10.3389/fchem.2023.1016193. eCollection 2023. Front Chem. 2023. PMID: 36970405 Free PMC article.
-
Simultaneous quantification of co-administered trastuzumab and pertuzumab in serum based on nano-surface and molecular-orientation limited (nSMOL) proteolysis.RSC Adv. 2024 Jun 18;14(27):19550-19559. doi: 10.1039/d4ra03060e. eCollection 2024 Jun 12. RSC Adv. 2024. PMID: 38895524 Free PMC article.
-
Development of an LC-TOF/MS Method to Quantify Camrelizumab in Human Serum.Molecules. 2024 Oct 14;29(20):4862. doi: 10.3390/molecules29204862. Molecules. 2024. PMID: 39459229 Free PMC article.
-
Regulated bioanalysis of antibody-drug conjugates using LC-MS.Bioanalysis. 2025 Apr;17(8):549-560. doi: 10.1080/17576180.2025.2490468. Epub 2025 Apr 9. Bioanalysis. 2025. PMID: 40205765 Review.
-
Changing the drug development and therapeutic paradigm with biologic drug combinations and bispecifics: How to choose between these two approaches?Clin Transl Sci. 2022 Sep;15(9):2096-2104. doi: 10.1111/cts.13345. Epub 2022 Jun 11. Clin Transl Sci. 2022. PMID: 35611545 Free PMC article. Review.
References
-
- Godara A, Siddiqui NS, Lee L, Toskic D, Fogaren T, Varga C, Comenzo R, et al. Combined use of two monoclonal antibodies in patients with systemic AL amyloidosis and cardiac involvement. J Clin Oncol. 2019;37(15). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
