Structural and biochemical characterization of a glutathione transferase from the citrus canker pathogen Xanthomonas
- PMID: 32744260
- PMCID: PMC7397488
- DOI: 10.1107/S2059798320009274
Structural and biochemical characterization of a glutathione transferase from the citrus canker pathogen Xanthomonas
Abstract
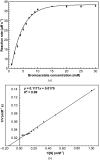
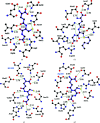
The genus Xanthomonas comprises several cosmopolitan plant-pathogenic bacteria that affect more than 400 plant species, most of which are of economic interest. Citrus canker is a bacterial disease that affects citrus species, reducing fruit yield and quality, and is caused by the bacterium Xanthomonas citri subsp. citri (Xac). The Xac3819 gene, which has previously been reported to be important for citrus canker infection, encodes an uncharacterized glutathione S-transferase (GST) of 207 amino-acid residues in length (XacGST). Bacterial GSTs are implicated in a variety of metabolic processes such as protection against chemical and oxidative stresses. XacGST shares high sequence identity (45%) with the GstB dehalogenase from Escherichia coli O6:H1 strain CFT073 (EcGstB). Here, XacGST is reported to be able to conjugate glutathione (GSH) with bromoacetate with a Km of 6.67 ± 0.77 mM, a kcat of 42.69 ± 0.32 s-1 and a kcat/Km of 6.40 ± 0.72 mM-1 s-1 under a saturated GSH concentration (3.6 mM). These values are comparable to those previously reported for EcGstB. In addition, crystal structures of XacGST were determined in the apo form (PDB entry 6nxv) and in a GSH-bound complex (PDB entry 6nv6). XacGST has a canonical GST-like fold with a conserved serine residue (Ser12) at the GSH-binding site near the N-terminus, indicating XacGST to be a serine-type GST that probably belongs to the theta-class GSTs. GSH binding stabilizes a loop of about 20 residues containing a helix that is disordered in the apo XacGST structure.
Keywords: GSTs; Xanthomonas; Xanthomonas citri subsp. citri; bromoacetate; citrus canker; dehalogenases; glutathione; glutathione S-transferases.
Figures



Similar articles
-
New genes of Xanthomonas citri subsp. citri involved in pathogenesis and adaptation revealed by a transposon-based mutant library.BMC Microbiol. 2009 Jan 16;9:12. doi: 10.1186/1471-2180-9-12. BMC Microbiol. 2009. PMID: 19149882 Free PMC article.
-
The gpsX gene encoding a glycosyltransferase is important for polysaccharide production and required for full virulence in Xanthomonas citri subsp. citri.BMC Microbiol. 2012 Mar 9;12:31. doi: 10.1186/1471-2180-12-31. BMC Microbiol. 2012. PMID: 22404966 Free PMC article.
-
Protein depletion using the arabinose promoter in Xanthomonas citri subsp. citri.Plasmid. 2017 Mar;90:44-52. doi: 10.1016/j.plasmid.2017.03.005. Epub 2017 Mar 24. Plasmid. 2017. PMID: 28343961
-
Xanthomonas citri subsp. citri surface proteome by 2D-DIGE: Ferric enterobactin receptor and other outer membrane proteins potentially involved in citric host interaction.J Proteomics. 2017 Jan 16;151:251-263. doi: 10.1016/j.jprot.2016.05.007. Epub 2016 May 11. J Proteomics. 2017. PMID: 27180281
-
Genetic and structural determinants on iron assimilation pathways in the plant pathogen Xanthomonas citri subsp. citri and Xanthomonas sp.Braz J Microbiol. 2020 Sep;51(3):1219-1231. doi: 10.1007/s42770-019-00207-x. Epub 2020 Aug 28. Braz J Microbiol. 2020. PMID: 31848911 Free PMC article. Review.
References
-
- Ahmad, S., Thulasingam, M., Palombo, I., Daley, D. O., Johnson, K. A., Morgenstern, R., Haeggström, J. Z. & Rinaldo-Matthis, A. (2015). Biochim. Biophys. Acta, 1854, 1365–1371. - PubMed
-
- Allocati, N., Casalone, E., Masulli, M., Ceccarelli, I., Carletti, E., Parker, M. W. & Di Ilio, C. (1999). FEBS Lett. 445, 347–350. - PubMed
-
- Allocati, N., Federici, L., Masulli, M. & Di Ilio, C. (2009). FEBS J. 276, 58–75. - PubMed
-
- Allocati, N., Federici, L., Masulli, M. & Di Ilio, C. (2012). Biochimie, 94, 588–596. - PubMed
-
- Armstrong, R. N. (1991). Chem. Res. Toxicol. 4, 131–140. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

