Activation of a neural stem cell transcriptional program in parenchymal astrocytes
- PMID: 32744501
- PMCID: PMC7440914
- DOI: 10.7554/eLife.59733
Activation of a neural stem cell transcriptional program in parenchymal astrocytes
Abstract
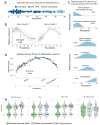
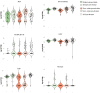
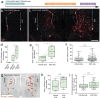
Adult neural stem cells, located in discrete brain regions, generate new neurons throughout life. These stem cells are specialized astrocytes, but astrocytes in other brain regions do not generate neurons under physiological conditions. After stroke, however, striatal astrocytes undergo neurogenesis in mice, triggered by decreased Notch signaling. We used single-cell RNA sequencing to characterize neurogenesis by Notch-depleted striatal astrocytes in vivo. Striatal astrocytes were located upstream of neural stem cells in the neuronal lineage. As astrocytes initiated neurogenesis, they became transcriptionally very similar to subventricular zone stem cells, progressing through a near-identical neurogenic program. Surprisingly, in the non-neurogenic cortex, Notch-depleted astrocytes also initiated neurogenesis. Yet, these cortical astrocytes, and many striatal ones, stalled before entering transit-amplifying divisions. Infusion of epidermal growth factor enabled stalled striatal astrocytes to resume neurogenesis. We conclude that parenchymal astrocytes are latent neural stem cells and that targeted interventions can guide them through their neuronal differentiation.
Keywords: astrocytes; mouse; neural stem cells; neurogenesis; neuroscience; regenerative medicine; single-cell RNA sequencing; stem cells.
Plain language summary
Regenerative medicine aims to help the body replace damaged or worn-out tissues, often by kick-starting its own intrinsic repair mechanisms. However, the brain cannot easily repair itself, and therefore poses a much greater challenge. This is because nerve cells or neurons, which underpin learning, memory, and many other abilities, are also the brain’s greatest weakness when it comes to tissue repair. In most parts of the adult brain, neurons are never replaced after they die. This means that damage to brain tissue – for example, after a stroke – can have severe and long-lasting consequences. Neural stem cells are one type of brain cell that can turn into new neurons if needed, but they are only found in a few parts of the brain and cannot fix damage elsewhere. More recent work in mice has shown that astrocytes, a common type of support cell in the brain that help keep neurons healthy, could also generate new neurons following a stroke. However, the ability was restricted to small numbers of astrocytes in a specific part of the brain. Here, Magnusson et al. set out to determine the molecular mechanisms behind this regenerative process and why it is unique to certain astrocytes. The researchers used a technique called single-cell RNA sequencing to analyze the genetic activity within individual mouse astrocytes that had been exposed to conditions mimicking a stroke. This method revealed which genes are switched on or off, thus generating a profile of gene activity for each astrocyte analyzed. This experiment showed that the profiles of astrocytes that had started to produce neurons were in fact nearly identical to neural stem cells. Even the astrocytes that could not generate neurons took the first few steps towards this genetic state; however, they stalled early in the process. Treating the brains of mice withepidermal growth factor, a powerful molecular signal that stimulates cell growth, kick-started nerve cell production in a subset of these cells – showing that at least some of the non-regenerative astrocytes could be stimulated to make neurons if given the right treatment. The results of this study shed new light on how some astrocytes in the brain gain the ability to form new neurons. In the future, this knowledge could help identify a source of replacement cells to regenerate the injured brain.
© 2020, Magnusson et al.
Conflict of interest statement
JM, MZ, GS, JM, MB, CT, BA, JF No competing interests declared
Figures











References
-
- Anderson KD, Alderson RF, Altar CA, DiStefano PS, Corcoran TL, Lindsay RM, Wiegand SJ. Differential distribution of exogenous BDNF, NGF, and NT-3 in the brain corresponds to the relative abundance and distribution of high-affinity and low-affinity neurotrophin receptors. The Journal of Comparative Neurology. 1995;357:296–317. doi: 10.1002/cne.903570209. - DOI - PubMed
-
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biology. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

